Figures & data
Figure 1. The structure of CIRP.
(A) The schematic structure of CIRP is shown, which contains an N-terminal RRM) and C-terminal RGG; The RNA-binding domain is composed of about 85 amino acids. (B) Amino acid sequence alignment and comparison of amino acid sequences of CIRP from different species. The CIRP protein sequence of human (Q14011), rat (Q60825) and mouse (P60824), bullfrog (Q9PTX2), xenopus laevis (093235) were acquired from Universal Protein Resource (Uniprot) databases.
CIRP: Cold-inducible RNA-binding protein; RGG: Arginine-rich region; RRM: RNA recognition motif.

Figure 2. The cellular response of intracellular CIRP and its role upon stress.
CIRP transcription and expression can be affected either upregulated or downregulated in response to various stress. CIRP is predominantly localized in the nucleus but can migrate to cytoplasm upon stress condition, and acts as an RNA chaperone regulating mRNA stability through its binding signature site in the 3′-UTR of its targets, which includes genes involved in DNA repair (ATR, RPA2), cellular redox metabolism (thiroredoxin), adhesion molecules (αE/β-catenin, C/E-cadherin), circadian mRNA (clock), reproduction-related genes in testis and TERT, response to hypoxia (HIF-1α), general translational machinery (eIF3H, eEF1A1, eIF4E-Bp1, eIF5A, and eIF4G3), and cardiac repolarization (α-subunits of Ito). In addition, CIRP can also be secreted into extracellular space through lysosome pathway upon stimulation by LPS or hypoxia/reoxygenation.
HIF-1α; Hypoxia-inducible factor 1α; TERT: Telomerase component.
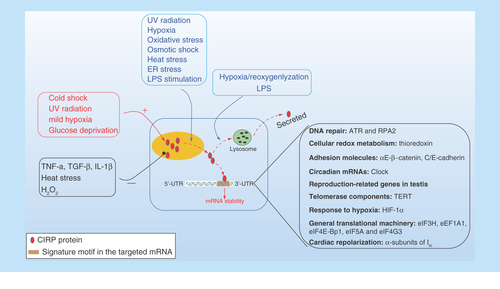
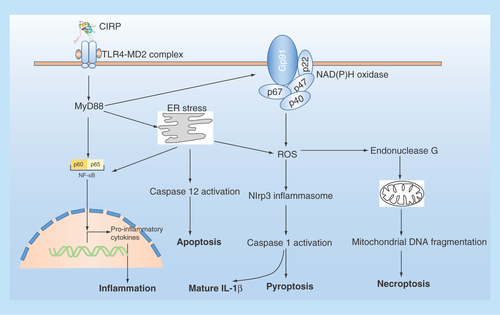
Figure 3. The extracellular role of CIRP in cells.
Extracellular CIRP can trigger multiple effects in cells via TLR4-MyD88 signaling pathway. ER stress: endoplasmic reticulum stress; NADPH oxidase is a multimolecular enzyme, composed of a membrane-associated 22-kDa α-subunit (p22phox) and a 91-kDa β-subunit (gp91phox, with cytosolic components composed of p47phox, p67phox and p40phox. Endonuclease G is a mitochondrion-specific nuclease that located in mitochondria.