Figures & data
Figure 1. Development of Agrobacterium rhizogenes-mediated transformation into Arabidopsis and tobacco hairy roots.
(A)Arabidopsis thaliana plants growing in containers. (B)Arabidopsis explants used for Agrobacterium rhizogenes infection. (C) Transgenic hairy root formation of Arabidopsis. (D, E & F) Transgenic hairy roots expressing the CaMV 35S-driven GUS and sGFP gene. (G) Hairy root formation of tobacco. (H & I) Excised tobacco hairy roots growth on solid and liquid medium. (J) Transgenic hairy roots of tobacco expressing the CaMV 35S-driven GUS gene. (K) Confocal laser scanning microscopy image of CaMV 35S-driven sGFP localization in tobacco transgenic hairy roots.
CaMV 35S: Cauliflower mosaic virus35S.
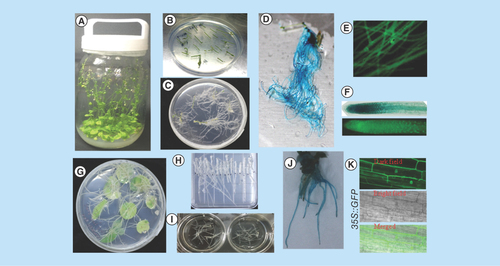
Table 1. Efficiency of hairy roots formation in different batch of transformation.
Figure 2. Transcript analysis of hairy and intact roots of Arabidopsis and tobacco exposed to rhizotoxic stress.
Expression of AtALMT1(A), RD29(B), and NtMATE(C), in wild-type hairy roots (only Agrobacterium Rhizogenes infection) and intact roots exposed to various rhizotoxic stress with (pH 5.0; [10 μM- : Arabidopsis, 30 μM- : tobacco Al, and 50 mM NaCl)] or without (pH 5.0; Al and NaCl) for 24 h was detected by RT (real-time)-polymerase chain reaction. Hairy roots were precultured for 3 days in MGRL solution containing 1% sucrose. Ubq1 and ACT1 were used as the internal reference gene. Error bars indicate ± standard deviation (n = 3). The primers used for qRT-polymerase chain reaction were similar used from previous studies of Sawaki et al. and Ohayama et al.
![Figure 2. Transcript analysis of hairy and intact roots of Arabidopsis and tobacco exposed to rhizotoxic stress.Expression of AtALMT1(A), RD29(B), and NtMATE(C), in wild-type hairy roots (only Agrobacterium Rhizogenes infection) and intact roots exposed to various rhizotoxic stress with (pH 5.0; [10 μM- : Arabidopsis, 30 μM- : tobacco Al, and 50 mM NaCl)] or without (pH 5.0; Al and NaCl) for 24 h was detected by RT (real-time)-polymerase chain reaction. Hairy roots were precultured for 3 days in MGRL solution containing 1% sucrose. Ubq1 and ACT1 were used as the internal reference gene. Error bars indicate ± standard deviation (n = 3). The primers used for qRT-polymerase chain reaction were similar used from previous studies of Sawaki et al. and Ohayama et al.](/cms/asset/e31ca3ca-3d70-4e8f-bfa4-2e5d4a38475f/ifso_a_12364167_f0002.jpg)
Figure 3. Protein localization in Agrobacterium rhizogenes-transformed hairy roots and cellular transformation.
(A) Fluorescent microscopic image of 35S:AtSTOP1:sGFP localization in hairy and intact roots of Arabidopsis. (B)35S:CcMATE1:sGFP localization in tobacco hairy roots and detection of cellular transformation base on GFP fluorescence. The confocal laser scanning microscopic image of CcMATE1 localized in cell plasma membrane of tobacco hairy roots (A) (dark field) and (B) (merged). Uniform and single cell transformation of CcMATE1 was detected based on GFP fluorescence.
GFP: Green fluorescent protein.
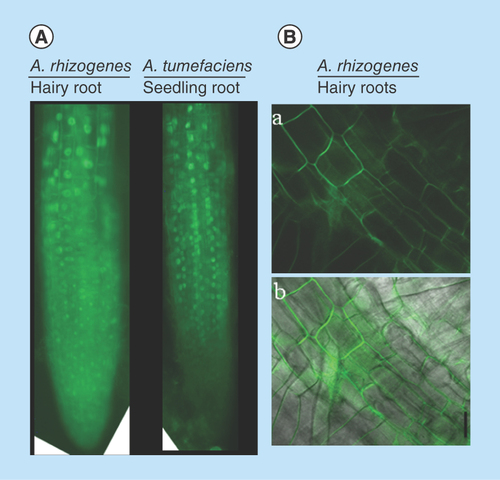
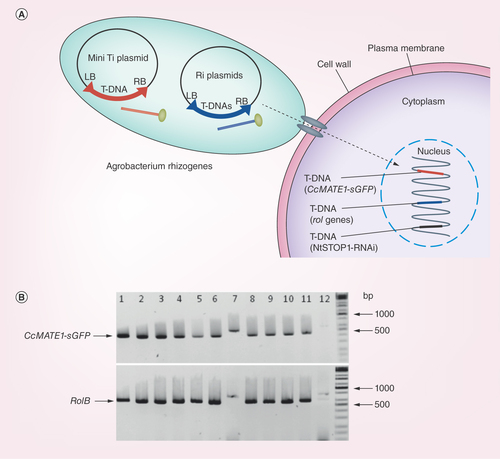
Figure 4. Diagrammatic model and co-transformation efficiency of Ri and mini Ti plasmid in Agrobacterium rhizogenes developed tobacco hairy roots.
(A) Diagrammatic model illustrating co-integration of T-DNAs from mini Ti and Ri plasmid into the hairy roots of NtSTOP1-KD tobacco. Transgenic Agrobacterium rhizogenes carries two Ti plasmid; (1) T-DNA of mini Ti plasmid carries CcMATE1-sGFP, kanamycin as antibiotic resistance marker gene; (2) T-DNA of Ri plasmid carries root locus (rol; rolA, rolB and rolC) gene, responsible for opine production. The T-DNA of NtSTOP1-KD tobacco host plant carries sequence of STOP1-RNAi as well as kanamycin and hygromycin resistance antibiotic marker genes. (B) Genomic DNA was extracted from roots transformed with CcMATE1-sGFP, and genes were amplified using primers flanking the target sequence. The rolB gene present in the Ri plasmid of A. rhizogenes and CcMATE1-sGFP present in mini Ti plasmid. Presence of amplicon of hairy roots marker gene (rolB; 670 base pair) and mini Ti plasmid caring GUS (500 base pair) showed co-transformation. Lanes 1–11, tobacco hairy roots; lane 12, negative control (NtSTOP1-KD tobacco). The primer pairs of 5′-TAGCCGTGACTATAGCAAACCCCTCC-3′ and 5′-GGCTTCTTTCTTCAGGTTTACTGCAG-3′, and 5′-TACCCCGACCACATGAAGCAG-3′ and 5′- TACTTGTACAGCTCGTCCATGC-3′, were used for amplification of RolB and CcMATE1-sGFP genes, respectively.
Ri: Root-inducing; Ti: Tumor-inducing.