Figures & data
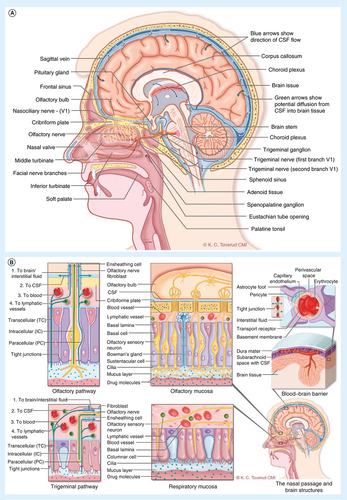
(A) Lateral section of the human nose and brain showing key anatomical structures and nerves relevant to drug transport from the blood to brain and from the nose to the brain. (B) The potential transport routes for substances into the brain from the blood across the blood–brain barrier and along the olfactory and the trigeminal pathways. In order to reach the neurons in the brain, substances in the blood must cross the tight endothelial barrier of the brain capillaries (the blood–brain barrier), or first cross the more 'leaky' barrier of the choroid plexus into the CSF and subsequently cross the barrier between the CSF and the brain interstitium (blood–CSF barrier). The olfactory filaments penetrate the nasal mucosa of the upper part of the nose and substances may be transported inside the nerve axon (intracellular). Transport across the mucosa also occurs between the cells (paracellular) or through the cells (transcellular). After crossing the mucosa substances may follow channels surrounding the nerve bundles (perineuronal), be absorbed into submucosal blood and lymphatic vessels or move into the subarachnoid CSF where they may enter the brain interstitum via perivascular channels. The trigeminal nerve endings do not penetrate the mucosal surface. Substances must cross the mucosa and continue along the same transport routes as described above. A part of the ophthalmic branch (V1) innervates the upper anterior nasal segment with similar projections as the olfactory nerve. The maxillary branch (V2) provides sensory and parasympathetic innervation to the majority of the respiratory mucosa and projects to the brain stem.
CSF: Cerebrospinal fluid.
Cross-sections of the complex nasal passages and endoscopic images highlighting the small dimensions and narrow geometry of the nasal valve, as well as its dynamics during nasal inhalation and when a positive pressure is applied. The target sites for N2B delivery are located in the upperconvoluted slit-like nasal passages beyond this narrow anterior nasal valve.
Reproduced with permission from [Citation132].
![Figure 2. The complex nasal anatomy and the nasal valve.Cross-sections of the complex nasal passages and endoscopic images highlighting the small dimensions and narrow geometry of the nasal valve, as well as its dynamics during nasal inhalation and when a positive pressure is applied. The target sites for N2B delivery are located in the upperconvoluted slit-like nasal passages beyond this narrow anterior nasal valve.Reproduced with permission from [Citation132].](/cms/asset/44ffeaa7-dd20-4cc8-a4aa-57e1082a4f98/itde_a_12366113_f0002.jpg)
Lateral and superior view of the nasal passages illustrating the Breath Powered Bi-Directional nasal delivery concept (see text for more detailed description).
Reproduced with permission from [Citation23,Citation132].
![Figure 3. Breath Powered™ Bi-Directional™ nasal delivery concept.Lateral and superior view of the nasal passages illustrating the Breath Powered Bi-Directional nasal delivery concept (see text for more detailed description).Reproduced with permission from [Citation23,Citation132].](/cms/asset/99a5ce25-ff04-448e-b120-86640f19b161/itde_a_12366113_f0003.jpg)
Multiuse Breath Powered powder device incorporating a standard size 3 capsule in disposable nosepiece unit and the Breath Powered multidose liquid device incorporating a standard spray pump with 120 doses.
Reproduced with permission from [Citation48,Citation132].
![Figure 4. Breath Powered™ Bi-Directional™ nasal delivery devices.Multiuse Breath Powered powder device incorporating a standard size 3 capsule in disposable nosepiece unit and the Breath Powered multidose liquid device incorporating a standard spray pump with 120 doses.Reproduced with permission from [Citation48,Citation132].](/cms/asset/5e5c6ca1-84d7-4ee2-9028-45d85caaa400/itde_a_12366113_f0004.jpg)
Initial regional gamma deposition (A) and horizontal distribution (B) 2 min after self-administration with conventional spray pump and Breath Powered™ Bi-Directional™ nasal powder device.
Reproduced with permission from [Citation48,Citation132].
![Figure 5. Initial regional gamma deposition data.Initial regional gamma deposition (A) and horizontal distribution (B) 2 min after self-administration with conventional spray pump and Breath Powered™ Bi-Directional™ nasal powder device.Reproduced with permission from [Citation48,Citation132].](/cms/asset/71c2e26a-600d-4ff9-86e6-35f5c42d52d1/itde_a_12366113_f0005.jpg)
Initial gamma deposition (0–2 min after delivery) in the same healthy subject following self-administration with a traditional spray and three Breath Powered™ device variants, the standard and the nose to brain variants of the multidose liquid device and the powder device shown in
N2B: Nose to brain.
Reproduced with permission from [Citation23,Citation48,Citation132].
![Figure 6. Initial gamma deposition in the same subject.Initial gamma deposition (0–2 min after delivery) in the same healthy subject following self-administration with a traditional spray and three Breath Powered™ device variants, the standard and the nose to brain variants of the multidose liquid device and the powder device shown in Figure 4.N2B: Nose to brain.Reproduced with permission from [Citation23,Citation48,Citation132].](/cms/asset/9269a38a-98de-4126-bf68-1af3bb47e173/itde_a_12366113_f0006.jpg)
Mean initial nasal gamma deposition in seven healthy subjects 2 min after self-administration with the Breath Powered™ nose to brain device variant. Approximately 30% and 80% of the delivered dose was deposited in the upper third and upper two-thirds, respectively. Approximately 15% was found in the lower third and a small fraction (∼5%) was deposited or had been cleared to the nasopharynx 2 min after delivery.
Reproduced with permission from [Citation23,Citation48].
![Figure 7. Breath Powered™ nose to brain powder device variant; initial horizontal distribution.Mean initial nasal gamma deposition in seven healthy subjects 2 min after self-administration with the Breath Powered™ nose to brain device variant. Approximately 30% and 80% of the delivered dose was deposited in the upper third and upper two-thirds, respectively. Approximately 15% was found in the lower third and a small fraction (∼5%) was deposited or had been cleared to the nasopharynx 2 min after delivery.Reproduced with permission from [Citation23,Citation48].](/cms/asset/c341bdad-e7e4-4405-a51e-bc8c4e1fcf2c/itde_a_12366113_f0007.jpg)
