Figures & data
Figure 1. CPT is an ERα modulator. (A) Chemical structure of estradiol and CPT. (B) Molecular model of estradiol and CPT docking with ERα. The protein structure is shown in plum color (ribbon), CPT and estradiol are shown in green and yellow carbon scheme, respectively. Hydrogen bonds are shown in blue dashed lines. (C) Direct binding of CPT to ERα in the ERα competitive-binding assay. Assay composition consisted of serial dilutions of the indicated compounds in 1% final DMSO concentration, 2 nM ERα-LBD, 3 nM Fluormone™ ES2 Green, and 2 nM Tb anti-GST antibody. Results are shown with the corresponding IC50 values. Experiments were repeated 3 times. Data shown are the mean ± SD.
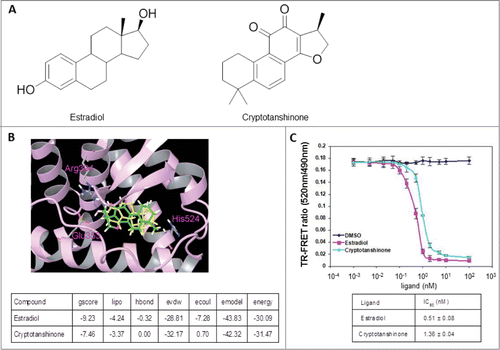
Figure 2. CPT inhibits ER-mediated transcriptional activity but not ERα protein levels. (A) CPT inhibits E2-induced ERE reporter gene activity. ZR-75-1 and MCF7 cells were transiently transfected with an ERE reporter construct or a control Renilla luciferase construct. After 24 h of transfection, cells were treated with various concentrations of CPT with 10 nM E2 or left untreated for 24 h. Results are shown as mean ± SD of 3 independent experiments. (B) ZR-75-1 and MCF7 cells were treated with or without 10 nM E2, 2 μM CPT or a combination of both for 24 h and then the mRNA expression of ER target genes (pS2, and Cat D) was analyzed using real-time RT-PCR. Data shown are mean ± SD of 3 independent experiments with 3 replicates in each experiment. (C) ZR-75-1 and MCF7 cells were treated with various concentrations of CPT with or without 10 nM E2. After treatment for 48 h, the cells were harvested and were analyzed for protein expressions of ER target genes (pS2, and Cat D) using Western blot. Experiments were repeated 3 times and similar results were obtained in all. Therefore, representative result from one experiment is shown.
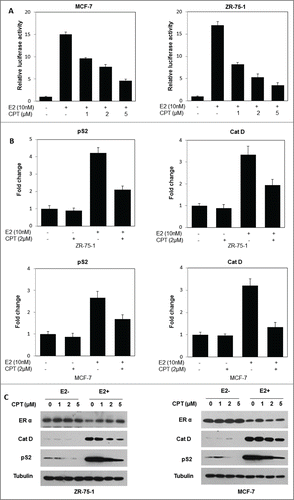
Figure 3. CPT inhibits estrogen-stimulated cell growth and survival in ERα-positive breast cancer cells. (A) ZR-75-1 and MCF7 cells were grown in phenol red-free DMEM supplemented with 10% charcoal/dextran without FBS for 24 h and then treated with various concentrations of CPT with or without 10 nM E2. After treatment for 48 h, cell growth was analyzed by CCK8 assay. Bars (mean ± SD) indicate the average of 3 independent experiments. (B) ZR-75-1 and MCF7 cells were plated in phenol red-free DMEM supplemented with 10% charcoal/dextran without FBS. When attached, the cells were treated with or without 10 nM E2, 0.2 μM CPT or a combination of both. Colonies were enumerated 10 days (ZR-75-1 cells) or 16 days (MCF7 cells) later and normalized against the number of colonies derived from control cells without CPT treatment. Data are shown as mean ± SD. Representative results from 2 independent experiments are shown.
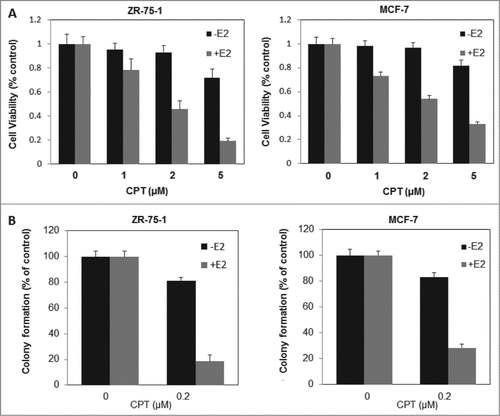
Figure 4. CPT inhibited breast cancer cell growth in an ER dependent manner. (A) IC50 of CPT in different breast cancer cells. We determined the IC50 values of CPT in ZR-75-1, MCF7, MDA-MB-231 and MDA-MB-435 cells. All cells were seeded on 96 well plates in phenol red-free DMEM supplemented with 10% charcoal/dextran without FBS for 24 h and then treated with a range of CPT concentrations alone or with 10 nM E2 for another 48 h. IC50 values were determined by CCK8 assay. Data represent mean ± SD of 3 independent experiments with 3 replicates in each experiment. (B and C) ERα knockdown suppressed the E2-induced transcriptional activity. Control and ERα-depleted ZR-75-1 and MCF7 cells were transiently transfected with an ERE reporter construct or a control Renilla luciferase construct. After 24 h of transfection, cells were treated with various concentrations of CPT with 10 nM E2 or left untreated for 24 h. Results are shown as mean ± SD of 3 independent experiments. (D) The inhibitory effect of CPT on cell growth was suppressed by silencing ERα. Control and ERα-depleted ZR-75-1 and MCF7 cells were grown in phenol red-free DMEM supplemented with 10% charcoal/dextran without FBS for 24 h and then treated with various concentrations of CPT in the presence of 10 nM E2. Two days later, cell growth was analyzed by CCK8 assay. Data are shown as mean ± SD of 3 independent experiments.
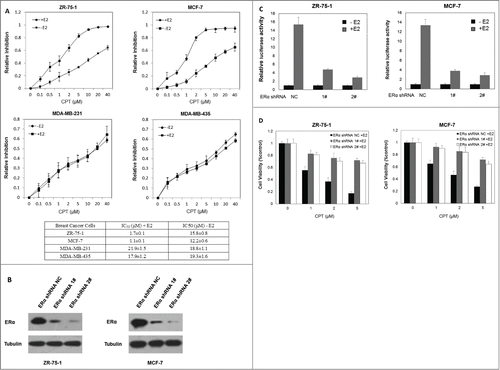
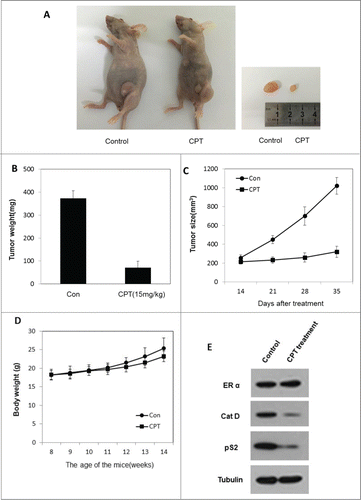
Figure 5. CPT effectively inhibited breast cancer growth in vivo. To test the therapeutic effect of CPT, 1 × 10Citation7 ZR-75-1 cells were injected into the abdominal mammary fat pad of 6-weeks old female BALB/c nude mice after implantation of estrogen pellets for 2 days. After 2–3 weeks, when tumor sizes were approximately 100 mm3, mice were randomly allocated to 2 experimental groups and intraperinoteally injected with control oil or 15 mg/kg CPT every 2 days for 3 weeks. The mice were then sacrificed and the tumors were collected for data analysis. (A and B) CPT inhibited tumor growth in the in vivo ZR-75-1 xenograft model. (C) CPT treatment inhibited ZR-75-1 tumor weights. Data are shown as mean ± SD (n = 6 for each group, P < 0.05). (D) CPT treatments had little effect on mouse body weights. Data are shown as mean ± SD (n = 6 for each group, P < 0.05). (E) CPT treatment inhibited the protein expression of ER target genes, pS2, and Cat D, in the in vivo xenografted ZR-75-1 tumors.