Figures & data
Figure 1. 3-Cl-AHPC mediated expression of miR-202 and miR-578 in pancreatic cancer cells. (A) miR-202 and miR-578 were down regulated in presence of 3-Cl-AHPC as demonstrated in microRNA array. (B). Changes in miRNAs levels were validated by quantitative Real time PCR. Cells were treated with 3-Cl-AHPC for 24 h. (C). 3′-UTR binding sites of miR-202 and miR-578 target genes Mxd1 and SAP18, respectively. (D, E). Increased expression of mRNAs of their target genes Mxd1 and SAP18 by SYBR-Green RT-PCR. Methodologies were as described in Materials and Methods. Error bars represent the mean of 3 separate determinations ± standard deviation (SD). *, ** and *** (<0 .05, <0.01 and <0 .001) indicate significantly differences between control and treated samples using the t-Test.
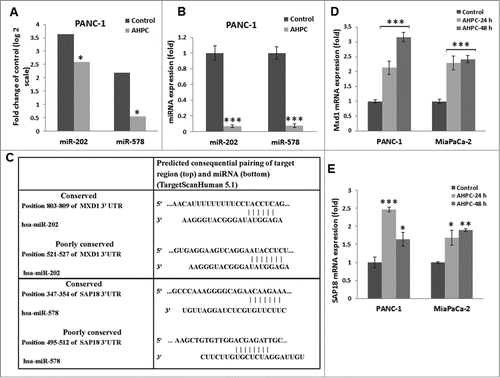
Figure 2. Overexpression of pre-miR-202 blocked the expression of its target protein Mxd1 mRNA and decreased Mxd1-3′-UTR activity. (A, B) Exposure of PANC-1 cells to 3-Cl-AHPC increased Mxd1 and SAP18 proteins levels as demonstrated by Western blots analysis and miR-578 miRNA mimic decreased SAP18-3′-UTR luciferase activity in cells (C, D). Increased expression of pre-miR-202 expression vector and pre-miR-202 reduced expression of Mxd1 mRNA in pre-miR-202 stably transfected cells. (D) Over-expression of pre-miR-202 blocked 3-Cl-AHPC mediated mRNA expression of Mxd1. (E) miR-202 decreased Mxd1-3′-UTR activity in transiently transfected cells. Error bars represent the mean of 3 separate determinations ± the standard deviation (SD). *, ** and *** (<0 .05, <0 .01 and <0 .001) were significantly differences between control and treated samples and •• was significantly different between 3-Cl-AHPC treated vector and pre-miR-202 expressing cells using the t-Test.
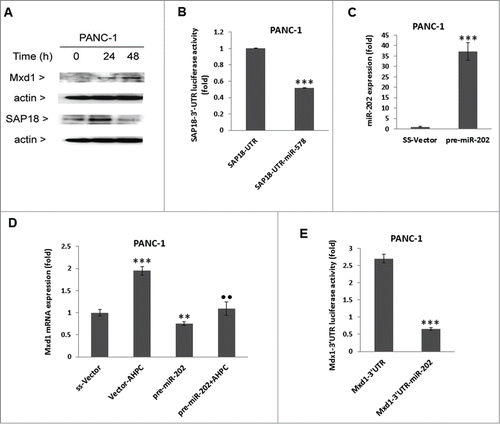
Figure 3. Inhibitor of miR-202 (OME-202) blocked the miR-202 mediated decrease of Mxd1 and pre-miR-202 overexpression induced apoptosis in PANC-1 cells. (A) Inhibitor 2′-O-methylated miR-202 (OME-202) inhibited miR-202 expression in transiently transfected cells. (B) OME-miR-202 alone and in the presence of 3-Cl-AHPC increased Mxd1 mRNA levels. Cells were transiently transfected with OME-miR-202 and then treated with 1 μM 3-Cl-AHPC for 24 h. (C) pre-miR-202 expression blocked the apoptosis in cells. The apoptosis induction was measured by cytoplasmic histone-associated-DNA-fragments using ELISA (enrichment factor = OD of PDCD5 expressed lysate / OD of vector lysate, OD at 405 nm-490 nm). Error bars represent the mean of 3 separate determinations ± SD. All pre-miR -202 expressed samples are significantly different from miR-vector. **, ***, and ••• (<0 .01 and <0 .001, respectively) were significantly different between control vehicle, ss-vector and ••• represented comparison between mi-202 to miR-202 + OME-202 treated samples.
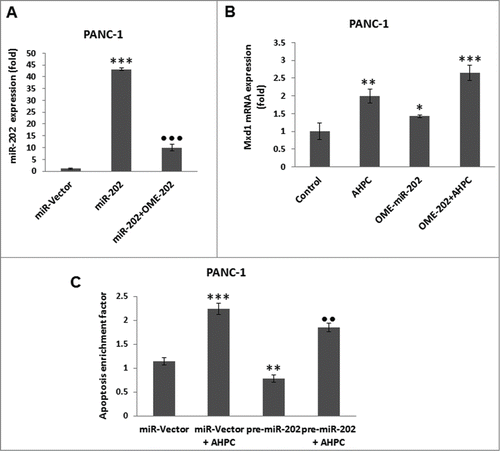
Figure 4. Mxd1 recruited Sin3A repressor complexes in the presence of 3-Cl-AHPC and heterodimerized with Max protein. (A) 3-Cl-AHPC increased Mxd1 protein binding with Sin3A and HDAC2 as demonstrated by co-immunoprecipitation. (B) 3-Cl-AHPC enhanced HDAC activity in cells. (C) Mxd1 increased its binding with Max protein whereas that of c-Myc with Max decreased in nuclear extracts of 3-Cl-AHPC treated cells. (D) 3-Cl-AHPC increased Mxd1 protein binding to the hTERT promoter whereas that of c-Myc to the hTERT promoter was concomitantly decreased as demonstrated by CHIP assay. (E) Mxd1 protein binding decreased in c-Myc promoter. Error bars represent the mean of 3 separate determinations ± SD. *, ** and *** (<0 .05, < 0.01 and <0 .001, respectively) were significantly between control and treated cells using the t-Test
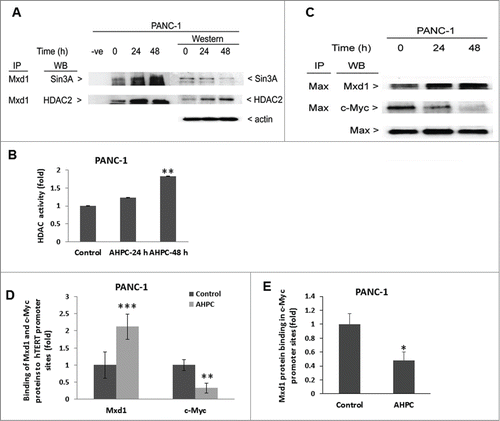
Figure 5. 3-Cl-AHPC decreased hTERT activity in pancreatic cancer cells. (A, B) 3-Cl-AHPC decreased hTERT mRNA and protein expression. (C, D) 3-Cl-AHPC decreased telomerase activities in cells. Error bars represent the mean of 3 separate determinations ± SD. *, **, and *** (<0 .05, <0 .01 and <0 .001) were significantly different in the treated samples compared to control using the t-Test.
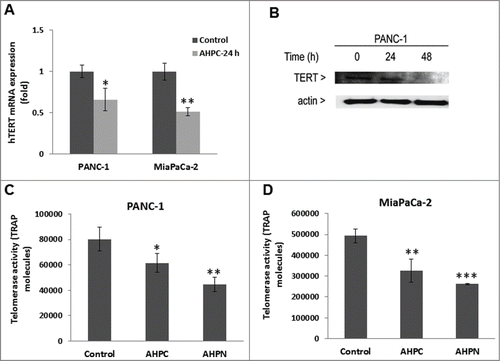
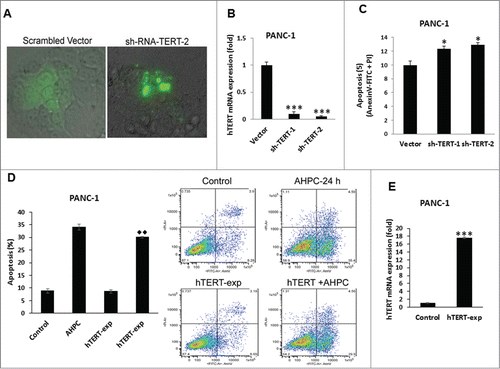
Figure 6. hTERT depletion is essential for induction of apoptosis in PANC-1 cells. (A) Knockdown of TERT led to nuclear fragmentation at 72 h of transfection in PANC-1 cells as determined by Phase Contrast Microscopy. (B) hTERT mRNA expression decreased in stably transfected sh-TERT knockdown cells. (C) sh-RNA-TERT knockdown cells induced apoptosis in transiently transfected cells. (D) Overexpression of hTERT blocked 3-Cl-AHPC mediated apoptosis. The apoptosis induction was measured by flow cytometry using Annexin V-FITC binding together with propidium iodide (PI) staining. Percentage of apoptotic cells corresponds to lower right (early apoptotic cells, annexin V positive, PI-negative) quadrants (right panel). (E) Increased hTERT mRNA expression after 72 h in over-expressing hTERT cells. Error bars represent the mean of 3 separate determinations ± SD. * and *** (<0 .05, and <0 .001) indicate significantly differences between vector and sh-TERT knockdown cells and ♦♦ (<0 .01) indicate significantly difference apoptosis between 3-Cl-AHPC treated and hTERT overexpressing cells using the t-Test.