Figures & data
Figure 1. TAZ is highly expressed in basal/triple-negative breast cancer (TNBC) patients. (A) Heat map and heirarchical agglomerative clustering showing Hippo-FIN activity among different breast cancer subtypes using RNA-Seq data sets from a TCGA patient panel (n = 515 patients) that consists of basal-like, Her2 enriched, Luminal A and Luminal B BC subtypes using the PAM50-defined subtype predictor as a classification metric. (B) Alterations in the Hippo-FIN are mutually exclusive. Integrated analysis of mRNA, mutation and copy number events identify TAZ, FRMD6, LATS1 and WWC1 genes as deregulated in basal-like breast cancer tumors to a maximum p-value of 0.05 by Fisher's exact test. Tumor samples are shown in columns and genes in rows. Only samples with >4 % alterations are shown. Shown are genes with statistically significant levels of: (i) mutation (MutSig, false discovery rate <0.1) and mutation types, (ii) deletions and amplifications for genomic regions with statistically significant focal copy number changes (GISTIC2.0) and (iii) RNA expression level for selected genes, expressed as fold change from the median value for all patient tumor samples. (C) TAZ protein is highly expressed in triple-negative breast cancer (TNBC) TMAs. Representative examples of TNBC TMA are shown. Upper, H&E staining; bottom, IHC staining exhibiting high TAZ nuclear expression. (D) TAZ expression in different types of breast cancer cells was revealed by immunoblot. β-Actin was used as loading control. (Upper panel) A population of MDA-MB-231 cells was infected with a pooled shRNA library of a subset of Hippo pathway genes. Log2 median fold change in shRNA abundance of experimental or control (neutral) shRNAs at day 0 vs day 21 tumors (n = 3). The frequency of shRNA-encoding constructs was determined by deep sequencing. An enrichment score was calculated for each shRNA using the probability distribution of the rank product statistics for replicated experiments.
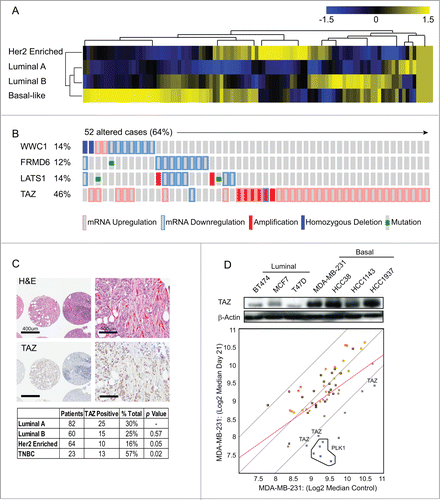
Figure 2. TAZ induces the breast cancer stem cell (CSC) properties and breast tumor formation. (A) Schematic of human TAZ protein showing the TEAD-interaction domain (TEAD-B), WW domain (WW), transcription activation domain (TAD) and PDZ-binding motif. Four serine–to-alanine point mutations (S66A, S89A, S117A and S311A) are introduced into wild-type TAZ construct (TAZ-4SA); additional serine-to-alanine mutation (S51A) is introduced into TAZ-4SA construct (TAZ-4SA-S51A), which leads to the loss of interaction of TAZ with TEAD. (B) Ectopic expression of constitutively active TAZ-4SA in human non-transformed breast epithelial MCF10A cells as revealed by immunoblot. β-Actin was used as the loading control. (C) Images and quantifications of mammosphere formation of vector or TAZ-4SA transduced MCF10A cells. Bars denote standard errors (n = 6). Representative images are shown. (Scale bar, 100μm). (D) Flow cytometry analysis of CD44high/CD24low population in vector or TAZ-4SA transduced MCF10A cells. Percentage of CD44high/CD24low subpopulation is indicated. (E) Quantification of mammosphere formation in vector or TAZ-4SA transduced HMEC cells. Bars denote standard errors (n = 6). Insert: Ectopic expression of constitutively activate TAZ-4SA in human breast epithelial HMEC cells was revealed by immunoblot. β-Actin was used as the loading control. (F) TAZ-4SA-transduced MCF10A cells induce mammary tumor formation when injected into the mammary fat pad of NOD/SCID mice (n = 6). (G) Histological analysis of tumors from the TAZ-4SA injected mice. Shown are H&E and IHC staining of Ki67, pan-cytokeratin AE1/3 and human-specific vimentin.
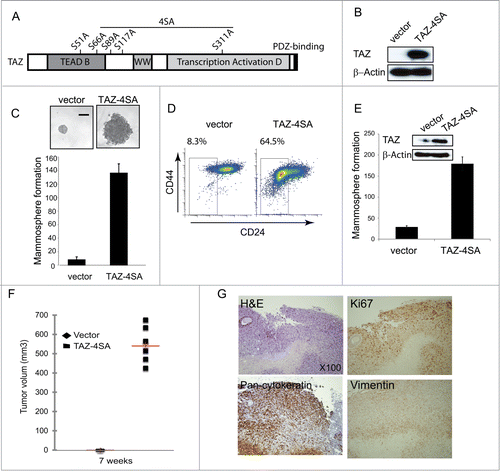
Figure 3. TAZ-induced breast CSC properties are dependent on the TAZ-TEAD interaction. (A) Ectopic expression of TAZ-4SA or TAZ-4SA-S51A in human breast epithelial MCF10A cells as revealed by immunoblot. β-Actin was used as the loading control. (B) Quantification of mammosphere formation in TAZ-4SA or TAZ-4SA-S51A transduced MCF10A cells. Bars denote standard errors (n = 6). (C) Flow cytometry analysis of the CD44high/CD24low population in vector, TAZ-4SA or TAZ-4SA-S51A transduced MCF10A cells. Percentage of CD44high/CD24low subpopulation is indicated. In contrast to TAZ-4SA, TAZ-4SA-S51A fails to increase the CD44high/CD24low cell subpopulation. (D) Real-time RT-PCR examination of TEAD1, TEAD2, TEAD3 and TEAD4 mRNA followed by treatment with control or 2 independent shRNAs (shTEAD#1, 2) in the TAZ-4SA-transduced MCF10A cells. GAPDH was used as an internal control (**, p < 0 .001). (E) Flow cytometry analysis of the CD44high/CD24low population in control, shTEAD#1 or shTEAD#2 treated cells.
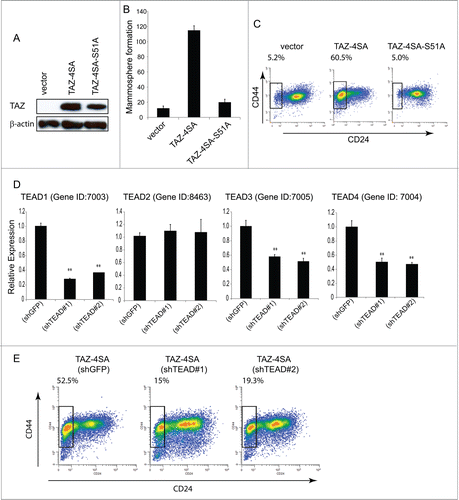
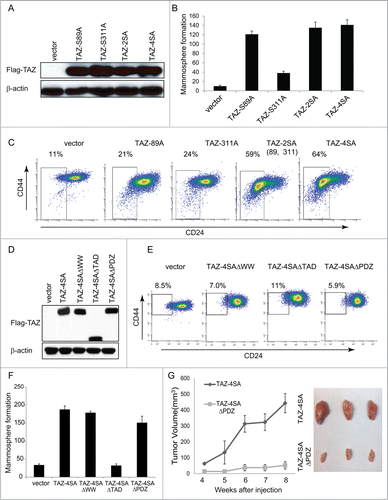
Figure 4. For figure legend, see page 166.Figure 4 (See previous page). The WW and TAD domains are important for TAZ induced mammary tumor formation. (A) Ectopic expression of TAZ-S89A, TAZ-S311A, TAZ-2SA (S89A, S311A), TAZ-4SA in human non-transformed breast epithelial MCF10A cells as revealed by immunoblot. β-Actin was used as the loading control. (B) Quantifications of mammosphere formation of vector or TAZ-S89A, TAZ-S311A, TAZ-2SA (S89A, S311A) and TAZ-4SA transduced MCF10A cells. Bars denote standard errors (n = 6). (C) Flow cytometry analysis of CD44high/CD24low population in vector or TAZ-S89A, TAZ-S311A, TAZ-2SA (S89A, S311A) and TAZ-4SA transduced MCF10A cells. Percentage of CD44high/CD24low subpopulation is indicated. (D) Ectopic expression of TAZ-4SA, TAZ-4SAΔWW, TAZ-4SAΔTAD or TAZ-4SAΔPDZ in human non-transformed breast epithelial MCF10A cells as revealed by immunoblot. β-Actin was used as the loading control. (E) Flow cytometry analysis of CD44high/CD24low population in vector, TAZ-4SAΔWW, TAZ-4SAΔTAD and TAZ-4SAΔPDZ transduced MCF10A cells. Percentage of CD44high/CD24low subpopulation is indicated. (F) Quantifications of mammosphere formation of vector, TAZ-4SAΔWW, TAZ-4SAΔTAD, TAZ-4SAΔPDZ and TAZ-4SA transduced MCF10A cells. Bars denote standard errors (n = 6). (G) TAZ-4SA and TAZ-4SAΔPDZ-transduced MCF10A cells induce mammary tumor formation when injected into the mammary fat pad of NOD/SCID mice (n = 6). Representative images of induced mammary tumors are shown (Right panel).