Figures & data
Figure 1 (See previous page). High resolution chromatin immunoprecipitation analysis at the lamin B2 origin of DNA replication. (A) Schematic representation of the position of the 12 overlapping PCR segments, used for ChIP analysis, across the 1.1 kbp DNA region containing the start site of the Lamin B2 origin of DNA replication (highlighted in gray) and the TIMM13 promoter (purple) regions. B48 and B13 exemplify origin and the non-origin regions.Citation15 The promoter of the TIMM13 gene is located ∼250 bp downstream the LaminB2 start site and contains the binding sites for USF-1 (green box), SP-1 (red box) and NRF-1 (blue box) transcription factors. (B) Real time, quantitative PCR was used to assess the chromatin binding profiles of nucleosomal proteins (H2B and K14 acetylated H3), of 3 proteins known to interact with the start site and the TIMM13 promoter (ORC4, HOXC13 and USF-1), of the AP-1 proteins c-Fos, c-Jun and JunD, of NFAT-1 and NF-κB p65, and of the AP-1 cofactor PBX.
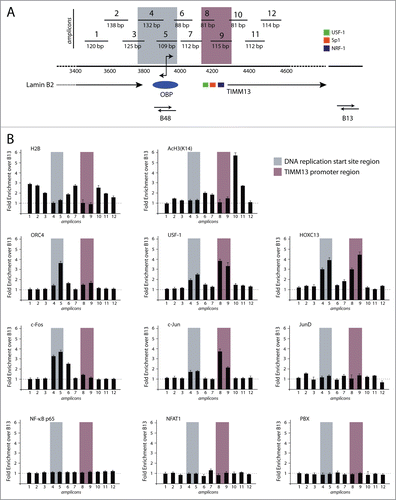
Figure 2. Co-immunoprecipitation analysis of proteins interacting with HOXC13. HA immunoprecipitation was performed with lysates from asynchronous crosslinked T98G cells overexpressing an HA-Flag tag HOXC13 construct using a specific anti-HA antibody for immuno-precipitation and the indicated antibodies for blottings. Lysates from asynchronous T98G cells served as a control. The NF-κB p65 subunit was used as a negative control for immunoprecipitation, whereas whole cell lysates (WCL) of both cell lines were used as positive controls. The asterisk indicates an unspecific signal detected by the anti-HOXC13 antibody.
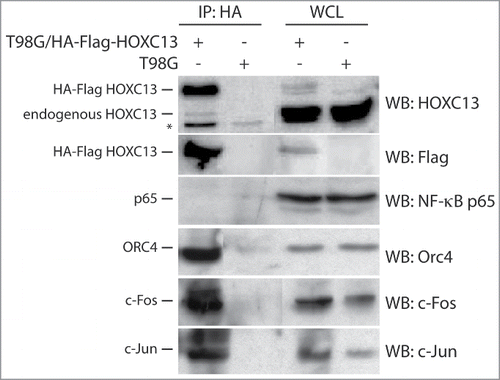
Figure 3. Spatial and temporal analysis of the localization of c-Fos and c-Jun proteins onto the origin DNA. (A) Flow cytometry profiles of T98G cells synchronized by 72 h serum starvation followed by the re-addition of serum. Cells were collected at 9, 14, 16 h and 20 h for mid-G1, late-G1, G1-S border and early S respectively. (B) Western blotting analysis of whole cell lysates (WCL) from each time point to detect the levels of c-Fos, c-Jun and Cyclin A. (C) Real time PCR analysis of chromatin immunoprecipitated at the different time points using the indicated antibodies. (D) Immunoblot using an anti ORC4 antibody performed on the samples used for the c-Fos and c-Jun ChIPs. The red dotted square indicates the co-presence of ORC4, mostly in late G1, in the c-Jun and c-Fos immunoprecipitated protein-complexes.
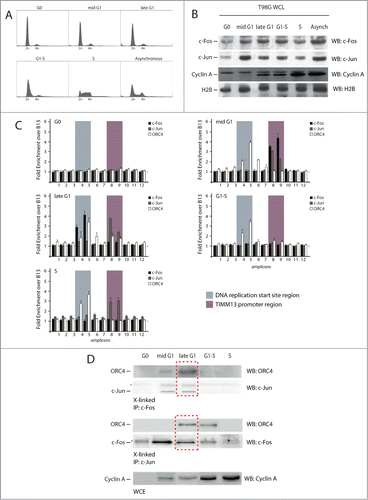
Figure 4. Sequential chromatin immunoprecipitation analysis (Re-ChIP) to detect interaction between AP-1 factors and ORC4 onto the Lamin B2 origin. (A) Schematic representation of the Re-ChIP procedure. Crosslinked chromatin, which was immunoprecipitated with anti-c-Fos or c-Jun antibodies, was subjected to a mild DTT treatment and re-probed using an anti ORC4 antibody. Finally, the immunocomplex was treated to reverse the crosslink and DNA analyzed by real time qPCR. (B) Upper part: flow cytometry profiles of T98G cells synchronized at different time points corresponding to different stages of the cell cycle, and subjected to Re-ChIP. Lower part: Results of real time qPCR analysis, showing that interaction between c-Fos, c-Jun and ORC4 occurs on the Lamin B2 origin only during the late G1 phase of the cell cycle. (C) Part of the material of each re-ChIP was subjected to immunoblot before qPCR to confirm the co-presence of c-Fos/ORC4 and c-Jun/ORC4.
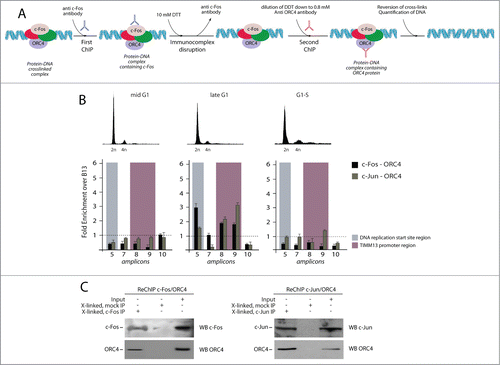
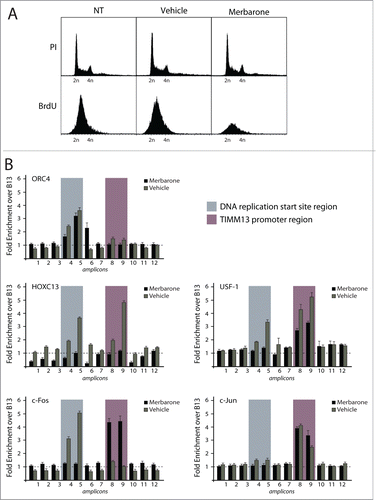
Figure 5. Effect of the topoisomerase II inhibitor merbarone on multi-protein complex assembly. (A) Flow cytometry analysis of T98G cells treated with merbarone. The upper panels show propidium iodide (PI) staining; the lower panels show BrdU incorporation. (B) Results of high resolution ChIP experiments in T98G cells treated with merbarone.