Figures & data
Figure 1. LANA2 reduces SUMO2 conjugation to p53 in vivo. (A) SUMO2 conjugated p53 decreases in both, HEK-293 cells (left panel) and MHH–PREB-1 cells (right panel) after expression of LANA2. (B) LANA2 does not have a general effect on SUMO2 conjugation process. (C) LANA2 does not significantly affect p53-SUMO2 conjugation in vitro. (D) LANA2 but not LANA1 inhibits p53-SUMO2 modification in HEK-293 cells.
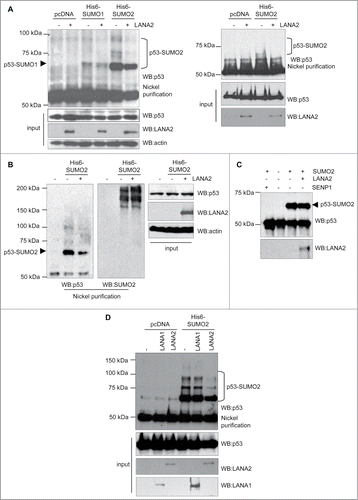
Figure 2. LANA2 requires intact SIM and SUMOylation domains to inhibit p53-SUMO2 conjugation, to interact with p53, and to inhibit senescence induced by type I interferon treatment or SUMO2 overexpression. (A) LANA2 requires intact SIM and SUMOylation domains to efficiently reduce p53-SUMO2 conjugation. (B) Interaction between p53 and LANA2 requires intact SIM and SUMOylation domains in LANA2. (C) Inhibition of SUMO2-induced senescence by LANA2-WT but not by LANA2 mutants in the SIM or SUMOylation domains. Senescence was determined by using a senescence β-galactosidase staining kit. The results are presented as mean of 3 independent experiments +/− SD and analyzed by Student's t-test (*, P < 0.05 versus cells co-transfected with SUMO2 and pcDNA). (D) Inhibition of senescence induced by interferon treatment by LANA2-WT but not by the mutants of LANA2 in the SIM or SUMOylation domains. H1299-p53 cells transfected with LANA2-WT, LANA2ΔSIM, LANA2ΔSUMO or LANA2TOTAL were analyzed by Western blotting (upper-left panel) and treated or not with 500 U/ml β-interferon for 4 d after which cell senescence was evaluated by β-galactosidase staining. Representative pictures of SA-β-Gal staining (upper-right panel) and percentage of SA-β-Gal-positive cells (lower panel) in response to interferon treatment. The percentage of positive staining was determined by dividing the number of β-Gal-positive cells into the total number within 10 random fields. The results are presented as mean of 3 independent experiments +/− SD and analyzed by Student's t-test (***, P < 0.0005 vs. pcDNA transfected cells).
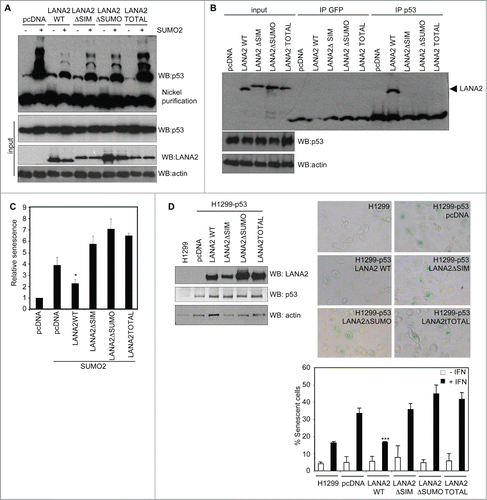
Figure 3. Regulation of p53-posttranslational modifications by KSHV proteins. While latent protein vCyclin induces phosphorylation of p53 at S33,Citation22 both latent proteins LANA1 and LANA2 inhibit phosphorylation of p53 at S15Citation3,23 and promote p53 ubiquitylation.Citation3,24 In addition, LANA2 also inhibits phosphorylation of p53 at S20,Citation3 acetylation of p53 at K320Citation3 and SUMO-2 modification of p53 (this report). The lytic protein vIRF1 inhibits phosphorylation of p53 at S15 and S392,Citation25 acetylation at K320 and K373 Citation25 and promotes p53 ubiquitylationCitation26; vIRF4 and K7 also induce p53 ubiquitylation,Citation7,27 and KbZIP induces SUMO2 conjugation of p53.Citation28