Figures & data
Figure 1. Germinal vesicle breakdown (GVBD) in severe DNA DSBs oocytes induced by zeocin. (A) Zeocin induced severe DNA DSBs at a high concentration. Fully grown GV oocytes were treated with 200 μg/ml zeocin for 1 h in M2 medium supplemented with 2.5 μM milrinone, while oocytes blocked for 1 h by milrinone were used as control. Oocytes were immunostained with anti-Pi-H2AX antibody immediately after 1 h treatment with or without zeocin (A), or after additional 5 h of IVM in M2 medium (B). (C) The GVBD rates were analyzed in the 2 groups from 2 h of IVM to 5 h of IVM. Asterisks indicate significant differences compared to the control group (P < 0.05).
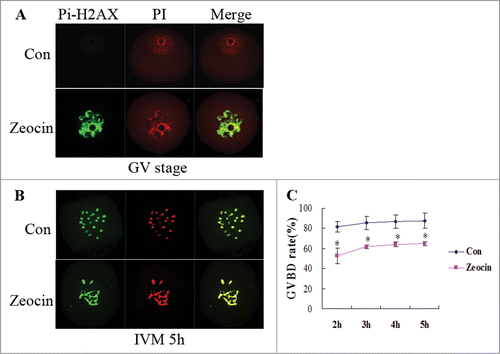
Figure 2. Severe DNA DSBs oocytes did not reach the MII stage at 12 h of IVM. (A) Oocytes with or without zeocin treatment were maturated in M2 medium for 12 h. (B) The percentage of PB1 extrusion in oocytes undergoing GVBD at 2 h of IVM was analyzed at 12 h of IVM. Two asterisks indicate dramatically significant difference compared to the control group (P < 0.01). (C) Spindle assembly and chromosome alignment at 12 h of IVM. Oocytes in the 2 groups were stained with anti-α-tubulin-FITC antibody and PtdIns. The percentages of each type are indicated.
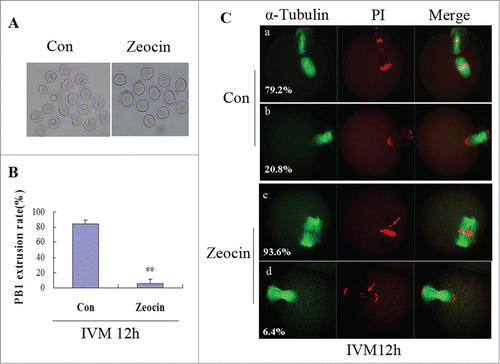
Figure 3. In vitro maturation of severe DNA DSBs oocytes was delayed. (A) PB1 extrusion rates were analyzed from 12 h of IVM to 18 h of IVM. Two asterisks indicate dramatically significant differences compared to the control group (P < 0.01). (B) Spindle assembly and chromosome alignment at 18 h of IVM in zeocin-treated oocytes. Oocytes were stained with anti-α-tubulin-FITC antibody and PI. The percentages of each type were indicated. (C) Chromosome spread of MII oocyte at 18 h of IVM in the DNA DSBs group. Chromosome fragments were observed after staining with anti-Bub3 antibody and PtdIns.
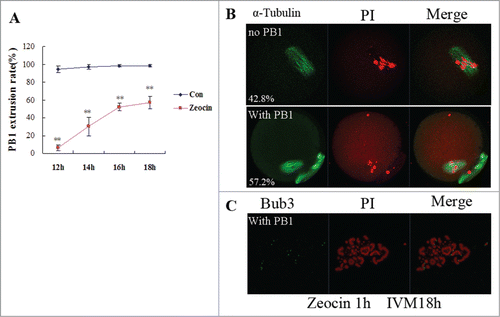
Figure 4. SAC activation and spindle microtubule-kinetochore attachment dsiruption by severe DNA DSBs. (A) Spindle assembly and chromosome alignment at the MI stage. Oocytes in the 2 groups were collected at 8 h of IVM, and then stained with anti-α-tubulin-FITC antibody and PI. Chromosome fragments ware misaligned in DNA DSBs oocytes at this stage (indicated by arrows). (B) Kinetochore-microtubule attachment check at the MI stage. Oocytes with or without zeocin treatment were cultured for 8 h in M2 medium followed by transfer into M2 medium pre-cooled to 4°C and incubated in a refrigerator at 4 °C for 10 min. Finally oocytes were stained with α-tubulin-FITC antibody and PtdIns. Kinetochore microtubules degraded in DNA DSBs oocytes. (C) SAC protein localization analysis at MI-AI stage. Oocytes at 9.5 h of IVM were harvested and chromosome spread was performed followed by immunostaining with anti-Bub3 antibody and PI. SAC was activated in DNA DSBs oocytes.
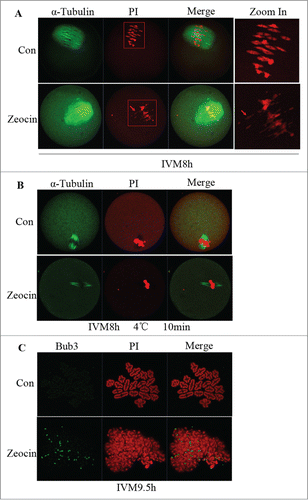
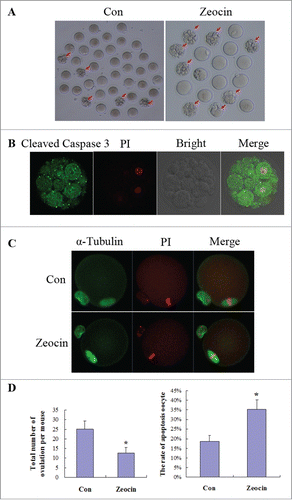
Figure 5. DNA DSBs induced oocyte apoptosis in vivo. (A) Superovulated oocytes in saline or zeocin treated mouse. Ten mice in each group were injected with 0.1 ml saline or 40 μg zeocin in 0.1 ml saline per day for 1 week. Then superovulation was performed. More fragmental oocytes (indicated by arrows) were observed in zeocin-treated mice. Representative oocytes ovulated from one mouse are displayed in (A). (B) Fragmented oocytes showed strong apoptosis signal. Fragmented oocytes were stained with anti-cleaved caspase 3 antibody and PtdIns. (C) Spindle assembly and chromosome alignment of superovulated non-fragmented oocytes. Superovulated non-fragmented oocytes were stained with anti-α-tubulin-FITC antibody and PI. Zeocin-treated mice appeared to ovulate MII oocytes with normal spindle assembly and chromosome alignment. (D) Analysis of total oocyte number of superovulation per mouse and apoptotic oocyte rate. Total number of ovulation per mouse was significantly reduced, and the rate of apoptotic oocytes was dramatically increased in the zeocin injection group. Asterisks indicate significant differences compared to the control group (P < 0.05).