Figures & data
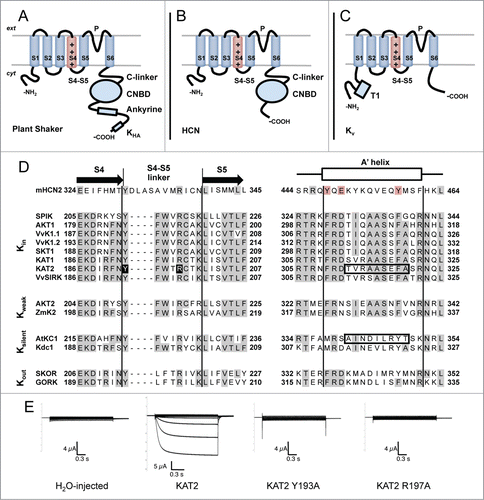
Figure 1 (See previous page). Membrane topology of plant Shaker, HCN and Kv channels. (A) Plant Shaker α-subunits exhibit a short N-terminal cytosolic tail, 6 transmembrane segments and a long C-terminal cytosolic tail. The fourth transmembrane segment (S4), which is rich in Arginines, acts as a voltage sensor. Between the fifth (S5) and the sixth (S6) transmembrane segment, there is a loop which participates in the formation of the pore (P) and contains the K+ selectivity filter. The C-terminal cytosolic tail consists of a C-linker, a cyclic-nucleotide binding domain (CNBD), an ankyrine domain and a KHA domain. (B) Animal HCN α-subunits share the same basic subunit structure with plant Shaker α-subunits with the exception of the post-CNBD region which is not conserved. (C) Animal Kv α-subunits display the same topology of plant Shaker and HCN α-subunits at the membrane level but the former notably differs in the cytosolic tails. The N-terminal tail is longer than in the other types of subunits and exhibits the tetramerization domain T1. The C-terminal tail is shorter and does not possess any of the domains described above. (D) Aminoacid sequence alignment of selected plant Shaker and mouse HCN2 α-subunits covering the S4-S5 linker and the beginning of the C-linker (putative A′ helix) regions which have been shown to interact and contribute to voltage-gating in HCN channels.Citation24,27 Plant sequences, previously characterized in heterologous systems, are grouped with respect to the voltage-gating properties of the corresponding channels (Kin: inward-rectifiers, Kweak: weak-rectifiers, Ksilent: electrically silent, Kout: outward-rectifiers). Sequence alignment was carried out with MUSCLE (MUltiple Sequence Comparison by Log-Expectation). Gray backgrounds depict identical residues in more than half of the analyzed sequences. Red background depicts conserved residues in the mouse HCN2 sequence. Black background depicts residues mutated in (E). Boxed sequences comprise residues exchanged in KAT2-AtKC1 chimeras described in Nieves-Cordones et al. 2014.Citation9 Protein alignment includes one sequence from mouse (mHCN2 (NP_032252.1)Citation43), 8 from Arabidopsis (SPIK (NP_180131.3),Citation44 AKT1 (NP_180233.1),Citation39 KAT1 (NP_199436.1),Citation45 KAT2 (NP_193563.3),Citation46 AKT2 (NP_567651.1),Citation47 AtKC1 (NP_974665.1),Citation10 SKOR (NP_186934.1)Citation48 and GORK (NP_198566.2)Citation49), 3 from grapevine (VvK1.1 (CAZ64538.1),Citation50 VvK1.2 (NP_001268010.1)Citation51 and VvSIRK (NP_001268073.1)Citation52), one from potato (SKT1 (NP_001275347.1)Citation10), one from maize (ZmK2 (NP_001105120.1)Citation53) and one from carrot (Kdc1 (CAB62555.1)Citation54). (E) Mutation of the conserved residues Tyr193 and Arg197 into Ala renders KAT2 channels electrically silent in Xenopus oocytes. Representative current traces recorded by 2-electrode voltage-clamp recordings in occytes injected with (from left to right): water, KAT2, KAT2 Y193A and KAT2 R197A in 100 mM K+ bath solution. Applied activation membrane voltages ranged from +40 to −170 mV (increments of 15 mV; holding potential, 0 mV; deactivation potential, −40 mV). Site-directed mutagenesis, cRNA preparation and solutions are described in Nieves-Cordones et al. 2014.Citation9