Figures & data
Figure 1. Propagation and transmission of misfolded SOD1. SOD1 misfolding can be induced by a variety of intracellular and extracellular stresses (1). Once a misfolded template is present, it can induce subsequent cycles of template-directed misfolding, converting neighboring native SOD1 molecules into pathological isoforms, which can subsequently form oligomers and aggregates over time (2). Presumably during the early stages of disease, misfolded SOD1 can also accumulate in the ER-Golgi system by an unknown mechanism (black box), where it can enter the vesicle-mediated secretory pathway, becoming selectively incorporated onto the outer surface of exosomes, exit the cell via secretion (3) and be taken up by neighboring cells. Alternatively, during later stages of disease when neural cells are injured and dying, large proteinaceous aggregates containing misfolded SOD1 are released (4) and subsequently taken up by neighboring cells via macropinocytosis (5). Uptake through this mechanism appears specific making the presence of certain cell surface receptors mediating uptake likely.
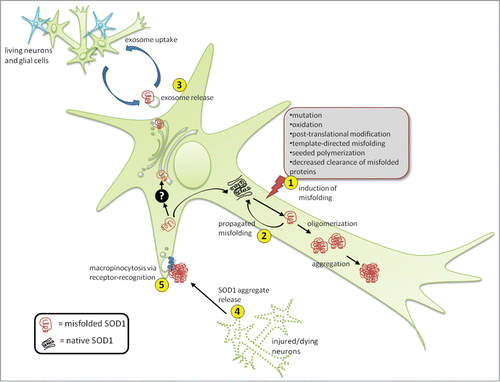
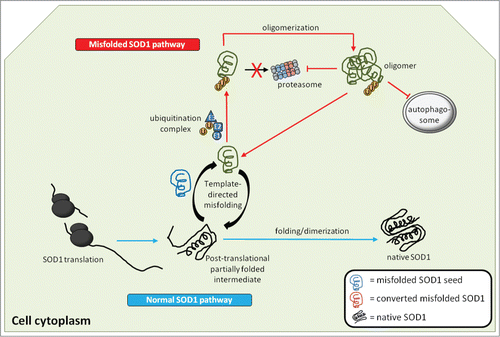
Figure 2. Generation and actions of misfolded SOD1 inside the cell. Native WTSOD1 or near-native SOD1 mutants are often highly protease resistant making their ability to misfold thermodynamically unfavourable. One explanation is that misfolded SOD1 seed (blue) utilizes post-translational intermediates of WT or mutant that remain partially unfolded and susceptible to induced pathological misfolding. Once populations of misfolded SOD1 (green) accumulate and begin to oligomerize, they become difficult to degrade via the ubiquitin-proteasome system, despite being conjugated with ubiquitin. It is also possible that misfolded SOD1 itself can act as an inhibitor of both the proteasome and autophagic systems, through as yet identified mechanisms, thereby allowing for aberrantly folded protein to elude proteostasis and build up within the cell.