Figures & data
Figure 1. Effect of HFIP and NH4OH pretreated synthetic Aβ42 on the yeast cells. 2 μM peptide was added to exponential phase C. glabrata (A) and S. cerevisiae (B) cells, suspended in water. Samples were incubated at 30°C for 48 h. Viability was determined as percentage of untreated cells (control). (* P < 0.05, ** P < 0.01, *** P < 0.005, and **** P < 0.001). Data are shown as Mean ± SEM.
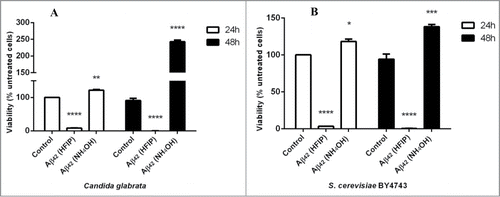
Figure 2. Effect of Aβ42 on the quiescent and non-quiescent fractions of C. glabrata. The quiescent daughter cells (A) were more resistant to Aβ42 prepared with HFIP method than non-quiescent mother cells (B) in the first 24 h of incubation. After 48 h of incubation both cell fractions were susceptible to the toxicity effect of Aβ42 (*** P < 0.005).
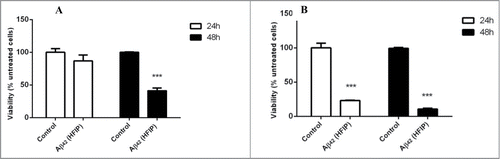
Figure 3. Staining of amyloid proteins present on S. cerevisiae cell walls by thioflavin T. First row (A–C) shows untreated S. cerevisiae cells in presence of ThT in comparison with cells treated with 5 μM NH4OH (D–F) and HFIP (G–I) prepared Aβ42 for 24 h in H2O. ThT staining comparison of untreated (control), NH4OH and HFIP pretreated cells are shown in panels A, D and G respectively. The HFIP pretreatment of Aβ42 results in higher fluorescence intensity and adhesion of peptide to the S. cerevisiae yeast cell wall protein. Scale bar = 50 μm.
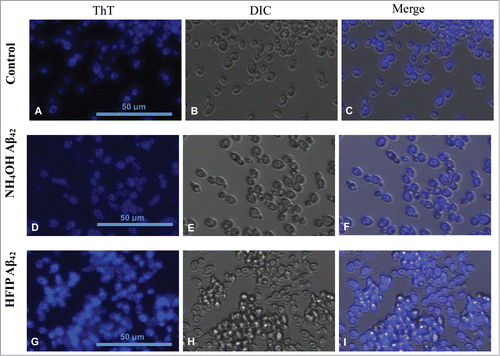
Figure 4. Flow cytometry analysis of S. cerevisiae treated with HFIP and NH4OH prepared Aβ42. The fluorescence intensity (FI) of ThT in the experimental samples was determined as a percentage of unstained cells. Yeast cells were grow to exponential phase, washed and resuspended in H2O. The cells were then treated with HFIP and NH4OH pretreated Aβ42 and incubated overnight along with control (untreated samples) at 30°C with shaking. The next day, cells were treated with 20 μM ThT and were analyzed by flow cytometry. Pacific blue filter was chosen to measure fluorescence. Unstained control cells (A), ThT stained control cells (B) and those treated with 5 μM of HFIP (C), and NH4OH pretreated Aβ42 have been shown.
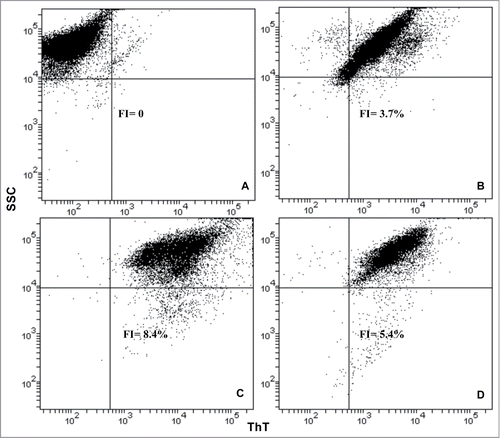
Figure 5. TEM analysis of peptide pretreated by NH4OH and HFIP at 37°C. The NH4OH method of oligomer formation (A) results in a higher amount of monomeric peptide compared with HFIP method in which still contain long fibrils (B). After 24 h of incubation the NH4OH method of pretreatment (C) induced a lower tendency form fibrils and those generated were shorter fibrils. The HFIP method (D) on the other hand resulted in a higher level of twisted and longer fibrils. The HFIP twisted fibril is shown (E). Scale bar = 200 nm.
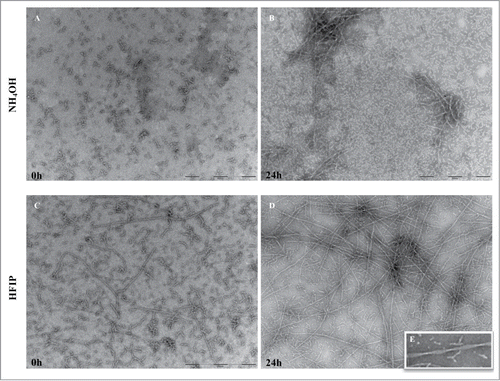
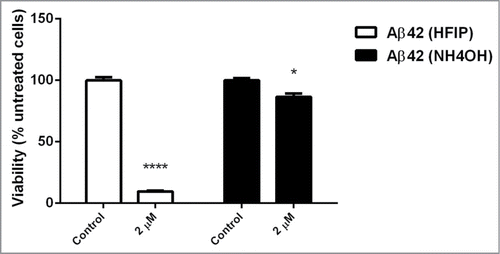
Figure 6. Effect of aged peptide on yeast cells. The NH4OH and HFIP pretreated Aβ42 peptide was incubated for 6 d at 37°C with no shaking. Exponentially growing S. cerevisiae were washed and suspended in H2O and then treated with 2 μM of these aged peptides and incubated overnight. Viability was determined the next day by transferring 100 μl of treated and untreated samples onto YEPD solid agar. (* P < 0.05 and **** P < 0.001).