Figures & data
Figure 1. Basic molecular domain structure of Eph receptors and ephrin ligands. Eph receptors have conserved structures and domains. TheN-terminal extracellular region consists of a ligand binding domain (LBD), an epidermal growth factor-like motif within a cysteine-rich domain (CRD) and two fibronectin-type III repeats (FN III1 and FN III2). The receptors pass through the membrane via a single transmembrane domain (TM). The intracellular C-terminus starts with a juxtamembrane region (JM), followed by a tyrosine kinase domain (TKD), sterile α motif (SAM) and a PDZ (postsynaptic density protein 95, discs large 1, and zonula occludens-1) binding motif. The ephrin ligands share a conserved extracellular, N-terminal receptor binding domain (RBD). Ephrin-A ligands are attached to the cell membrane with a glycosylphosphatidylinositol (GPI) anchor. In contrast, ephrin-B ligands have a C-terminal tail that extends into the cytoplasm of the ligand-bearing cell through a TM domain. The C-termini of ephrin-B ligands contain a cytoplasmic tail with a PDZ binding motif.
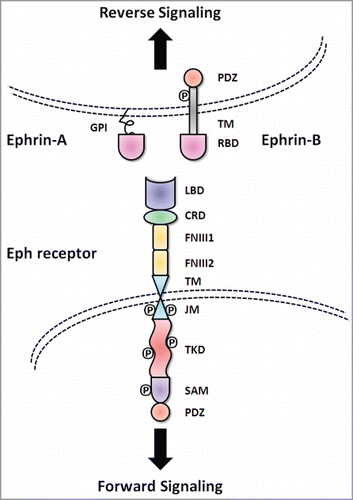
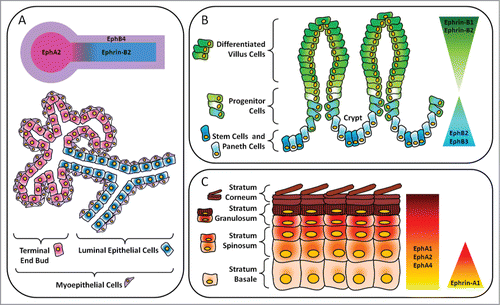
Figure 2. Layout of major Eph receptors and ephrin regulators in epithelial compartments. (A) Mammary gland. In the luminal epithelial cells lining the ducts, there is expression of ephrin-B2. Adjacent myoepithelial cells express EphB4. EphA2 is expressed in the terminal end bud. (B) Gastrointestinal tract. In the intestines, there is an inverse gradient of EphB and ephrin-B expression along the crypt-villous axis. EphB2 and EphB3 are important for maintenance of stem, progenitor and Paneth cells in the crypts. In the villi, ephrin-B1 and ephrin-B2 maintain the segregation of differentiated cells in the upper regions from precursor cells in the lower regions. (C) Epidermis. Ephrin-A1 is expressed in the progenitor layer, or stratum basale, of human epidermis while EphA1, EphA2 and EphA4 are present throughout all layers and are especially concentrated in the more differentiated suprabasal layers.