Figures & data
Figure 1. An illustration of ADAM domain structure. ADAMs consist of a prodomain (pro), metalloprotease domain (MP), disintegrin domain (D), cysteine-rich domain (C), EGF-like domain (except for ADAM10 and 17), a transmembrane domain and a cytoplasmic domain.
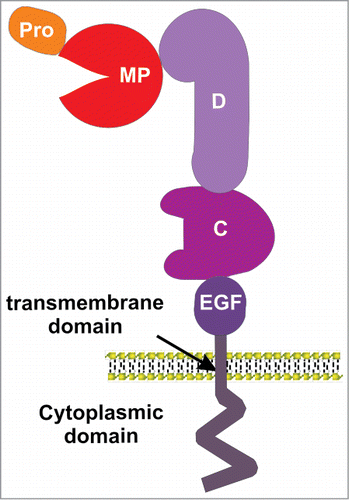
Figure 2. Proposed mechanisms of Eph/ephrin induced ADAM shedding. (A) Prior to Eph/ephrin interaction: Eph is in an inactive conformation, via interactions between the juxtamembrane and kinase domains; ADAM10 is poorly associated. (B) When Eph/ephrin interaction occurs between adjacent cells, the Eph/ephrin complex provide a new interface recognized by a substrate binding pocket in the C domain of ADAM10, positioning the protease domain to cleave ephrin from the opposing cell. Eph activation results in release of the inactive conformation and elongation of the EphA3 cytoplasmic domain, moving the kinase domain away from the plasma membrane, and facilitating ADAM10 association and ephrin cleavage. (C) Conformation changes in the ADAM C domain are proposed to occur via protein disulfide isomerase (PDI) activity, switching disufide bonds at a conserved CxxC motif. Upon activation of RTK receptors, reactive oxygen species (ROS) are produced by NOXs (NADPH-oxidases), which in turn inhibit PDI to favor an ‘active’ ADAM conformation, able to bind the Eph/ephrin complex, cleave ephrin and enable cell-cell retraction.
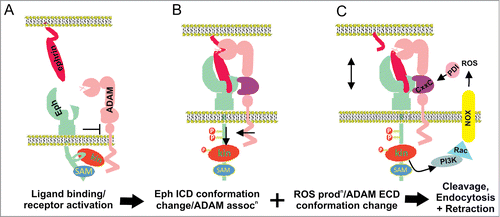
Figure 3. MMPs in Eph and/or ephrin shedding. (A) Ephrin-B1 ectodomain cleavage by MMP8, which is enhanced upon interaction with the EphB2 receptor.Citation99,100 Stimulation of ephrin-B1 by EphB2 activates the Arf1 GTPase, a critical regulator of membrane trafficking, most likely increasing the exocytosis of MMP8. The ephrin-B1 C-terminus also activates RhoA by binding to the adapter protein Dishevelled (Dsh), facilitating cell-cell repulsion and invasion. (B) Ephrin-B1-induced EphB2 receptor activation and its cleavage by MMP 2/9 cause RhoA activity via recruitment and subsequent activation of FAK, regulating growth cone withdrawal and collapse.Citation103 (C) Clusters of overexpressed EphA2 recruit MT1-MMP via its non-catalytic domains, mediating cleavage of EphA2 in cis at the fibronectin repeat type-III domain, stimulated by binding to ephrin-A1, and activating Src/Rho-mediated invasion.Citation107
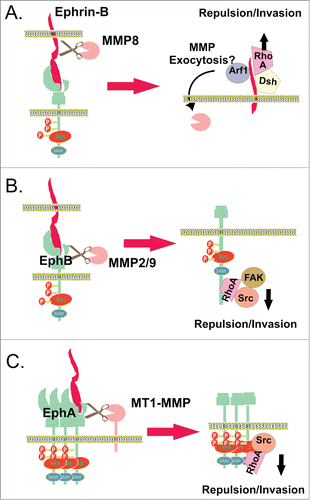
Table 1. Summary of protease function in Eph/ephrin signaling
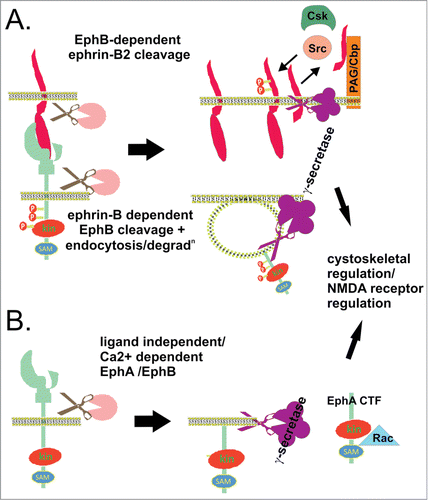
Figure 4. Regulated intramembrane proteolysis of Ephs/ephrins. (A) EphB/ephrin-B interaction can lead to sequential ectodomain and γ-secretase-mediated intramembrane proteolysis of both ligand and receptor in interacting cells. EphB2 bound ephrin-B2 ectodomain is cleaved by metalloprotease activity, generating a membrane bound cytoplasmic fragment (ephrin-B2-CTF1) which is further processed by γ-secretase, yielding a soluble cytoplasmic fragment (ephrin-B2-CTF2) (top cell). Inside the cell, ephrin-B2-CTF promotes tyrosine dephosphorylation of (PAG/Cbp), and relieves Csk-inhibition of Src kinases, which in turn facilitates tyrosine phosphorylation of ephrin-B2.Citation98,124 Ligand-binding to EphB2 also promotes receptor ectodomain cleavage, endocytosis, and γ-secretase cleavage, to produce soluble Eph cytoplasmic domain (bottom cell).Citation106 This regulated intramembrane proteolysis in both cells contributes to cytoskeletal regulation, migration, proliferation and survival. (B) EphA/B receptor ectodomain shedding also occurs in the apparent absence of ligand, via MMP/ADAM activity, stimulated by NMDA receptor signaling or calcim influx. The plasma membrane bound Eph cytoplasmic fragment (Eph-CTF) then undergoes γ-secretase cleavage. Both ligand-independent and -dependent EphB pathways may regulate NMDA receptor signaling and synaptic plasticity.Citation106 In addition, calcium-stimulated EphA4 shedding at synapses promotes dendritic spine formation via Rac signaling.Citation133