Figures & data
Figure 1. Effect of 1 hour PRP pre-treatment at 1/100 and 1/1000 dilution on SaOS-2 chemokinesis. The migration assay was performed in Boyden Chamber in 16 hours. Results are expressed as means of percentage of cells migrated versus control.
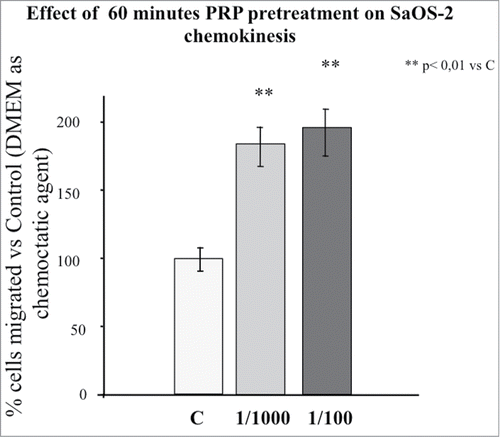
Figure 2. Effect of 1 hour PRP pre-treatment at 1/100 and 1/1000 dilution on SaOS-2 migration in presence of FBS 1% as chemotactic agent. The migration assay was performed in Boyden Chamber in 4 hours. Results are expressed as means of percentage of cells migrated versus control.
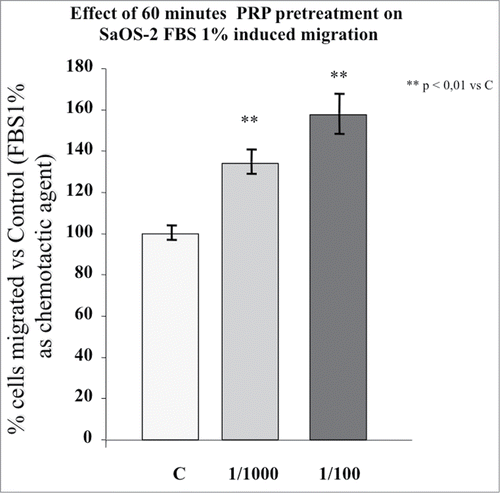
Figure 3. (A) Effect of a PRP treatment on the PDGF receptor α expression SaOS-2 osteoblasts. Cells treated with culture media, with PRP 1/100 or with PRP 1/1000 for 30, 60 or 150 minutes. (B) Effect of a PRP treatment on the PDGF receptor β expression SaOS-2 osteoblasts. Cells treated with culture media, with PRP 1/100 or with PRP 1/1000 for 30, 60 or 150 minutes.
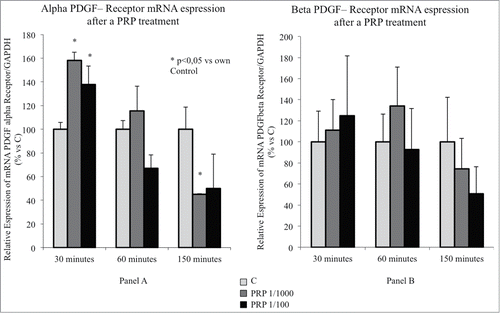
Figure 4. Effect of a PRP treatment on rearrangement of acin microfilaments. Images of phalloidin stained SaOS cells treated with culture media (A), with PRP 1/1000 for 30 minutes (B), with PRP 1/1000 for 60 minutes (C), with PRP 1/1000 for 150 minutes (D). Images in fluorescence microscopy at 63× magnification.
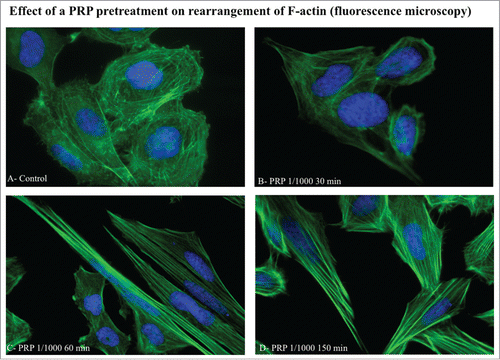
Figure 5. Effect of a PRP treatment on rearrangement of actin microfilaments. Fluorescence images of phalloidin stained SaOS-2 cells treated with culture media (A), PRP 1/1000 for 60 minutes (B) and PRP 1/1000 for 150 minutes (C). In the details (B and C) a lamellipodium rich in actin microspikes. Images in confocal microscopy at 63× magnification, zoom 2×.
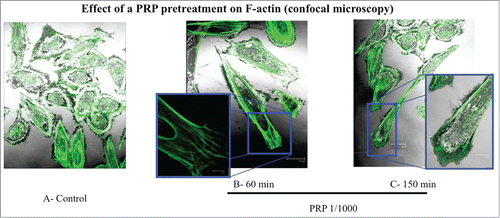
Figure 6. Phenotype composition of SaOS-2 cells after a 1 hour PRP treatment. Fluorescence images of the 3 types of SaOS-2 cells (Type I, Type II, and Type III) counted in control and PRP treated (for 1 hour) coltures. The number of cells is the mean of 6 fields for each treatment. Images in fluorescence microscopy at 10× magnification.
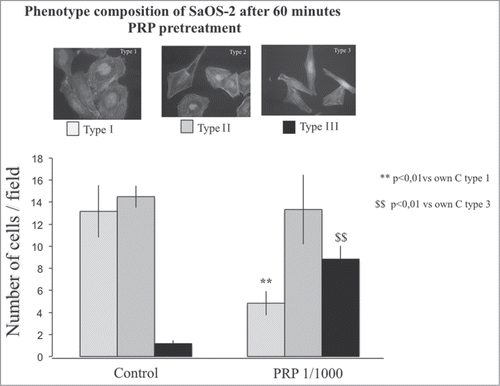
Figure 7. Effect of different doses of anti-PDGF-PRP pre-treatment on SaOS-2 migration in presence or in absence (DMEM) of FBS 1% as chemotactic agent. Before migration, cells were exposed for 1 hour to PRP at 1/1000 dilution. The migration assay was performed in Boyden Chamber in 4 hours. Results are expressed as means of percentage of cells migrated versus control.
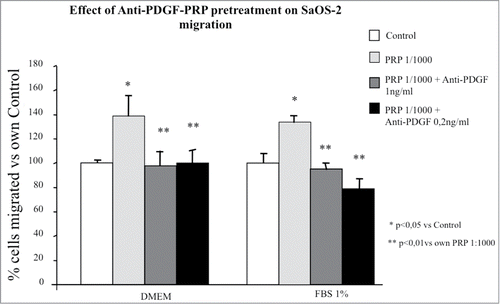
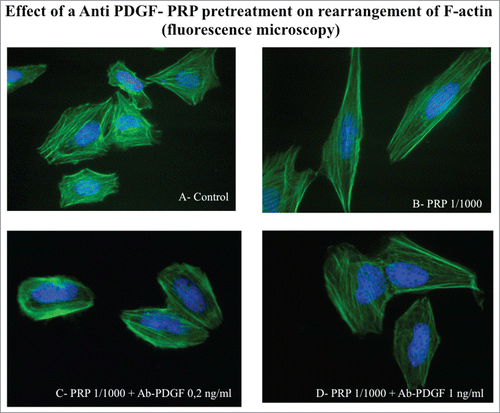
Figure 8. Effect of an Anti PDGF-PRP treatment on the rearrangement of actin microfilaments. Fluorescence images of phalloidin stained SaOS-2 cells treated with culture media (A), PRP 1/1000 for 60 minutes (B), PRP 1/1000 plus Ab PDGF 0.2 ng/ml (C) and PRP 1/1000 plus Ab PDGF 1 ng/ml and (D). Images in fluorescence microscopy at 63× magnification.