Figures & data
Figure 1. Microbiota and immune homeostasis in oral tolerance. (A) In the healthy gut there is a balance between beneficial bacteria and potentially harmful bacteria (pathobionts). This results in intestinal homeostasis with a balance between the pro-inflammatory Th1 and Th17 cells and regulatory T cells, allowing the generation of oral tolerance to food proteins such as gluten. (B) A disruption of early microbial colonization (C-section, neonatal antibiotic use) or a disruption of barrier function or microbial ecology (changes in diet, infections, or drugs) can results in intestinal dysbiosis. This can lead to imbalances between pro-inflammatory and regulatory immune cells. In genetically susceptible individuals, immune imbalances may promote loss of tolerance to food proteins, such as gluten.
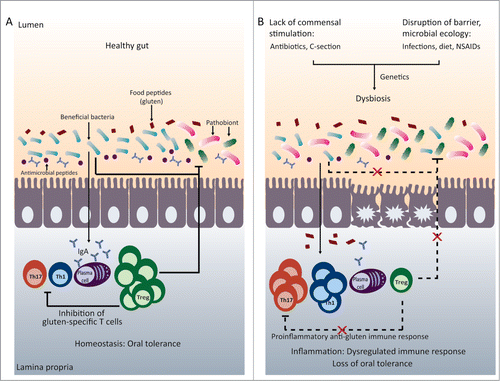
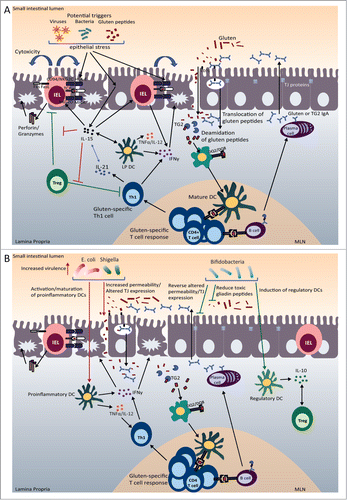
Figure 2. For figure legend, see next page.Figure 2 (See previous page). Celiac disease (CD) pathogenesis and potential microbial role as a disease modulator. (A) CD Pathogenesis. Gluten peptides in the small intestinal lumen translocate the epithelial barrier, either through paraceullular or transcellular mechansisms. Once in the lamina propria (LP), deamidation occurs, during which tissue transglutaminase (TG2) introduces negatively charged residues into the gluten peptides. Once gluten peptides are deamidated they can bind strongly and preferentially to DQ2/DQ8 molecules that are present on DCs. After migrating to sites of induction (mesenteric lymph nodes (MLN)), mature DCs present the gluten peptides to gluten-specific CD4+ T cells resulting in their activation and a gluten-specific Th1 response (production of IFNγ and IL-21). Gluten peptides and TG2 can also form complexes, which can be taken up by TG2 specific B cells or gluten-specific B cells. The presentation of gluten peptides by B cells to gluten-specific T cells results in B cell activation and the formation of anti-gliadin and anti-TG2 producing plasma cells. In the lumen, secretory anti-gliadin antibodies can bind gluten and transport gluten to the lamina propria via CD71-mediated transcytosis. Increased epithelial cell (EC) stress, triggered by gluten peptides, bacteria, or viruses, can upregulate stress molecules on epithelial cells (HLA-E, MICA/B) and induce IL-15 production from ECs. IL-15 can induce DC maturation and upregulate NK receptors (NKG2D) on IELs. The binding of NK receptors on IELs to their ligands (HLA-E and MICA/B) on ECs results in cytotoxic killing of ECs leading to tissue damage. IL-15 can also inhibit the regulatory effects of Tregs. (B) Pathogenic and protective role of microbiota in CD. Potential pathobionts, including E. coli and Shigella, may promote pro-inflammatory anti-gluten immune responses. First, E. coli strains isolated from CD patients were shown to have increased virulence. Second, E. coli and Shigella can induce the maturation of DCs and the production of pro-inflammatory cytokines (IL-12, TNFα) after gliadin stimulation. Finally, Shigella and E. coli can increase intestinal permeability and alter tight junction (TJ) protein expression. On the other hand, potentially beneficial bacteria, such as bifidobacteria, may reverse pathogenic, gluten-induced responses. Bifidobacteria can reverse gluten-induced increased permeability and altered TJ expression. Bifidobacterium species may also reduce the number of toxic, immunogenic gliadin peptides generated in the lumen. Finally, bifidobacteria can promote the production of IL-10 from DCs.