Figures & data
Figure 1. Cisplatin-treated GN-WT MEFs exhibiting SIGRUNB. (A) Expression of GFP-nucleolin in healthy GN-WT MEFs is confined to the nucleus. Hoechst 33342 was added to the culture medium of GN-WT MEFs and GFP-nucleolin and Hoechst fluorescence were monitored in live cells. GN-WT MEFs were treated with 25 μM cisplatin and after 15–24 h were subjected to live-cell imaging at 30-s intervals. (B) Appearance and subsequent rupture of GFP-nucleolin-containing nuclear bubble. The still images shown were taken from Vid. S1. Arrow indicates a bubble. The indicated time points (min:s) are expressed relative to the first image selected for presentation (t = 00:00), which corresponds to the image captured 34 min into the recording. (C) Stress-induced nuclear bubbles do not contain DNA. Hoechst 33342 was added to the culture medium 18 h after cisplatin treatment. Results shown (still images from time lapse imaging) are from the same field visualized separately for detection of GFP-nucleolin (upper panel) and Hoechst 33342 (lower panel). Dashed oval indicates the position of the nuclear bubble that lacks Hoechst 33342 staining. Indicated time points (min:s) are expressed as described in A. t = 00:00 corresponds to the image captured 43 min into the recording. The images shown are from representative experiments from 3, 8 and 8 independent experiments for A, B and C respectively.
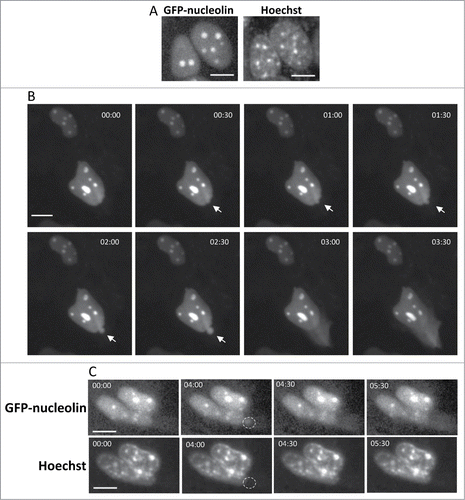
Figure 2. SIGRUNB preceded the appearance of typical late onset features of apoptosis (A) SIGRUNB occurs before the appearance of membrane blebbing and bubbling and nuclear condensation. The still images shown were taken from Vid. S2. Arrow indicates a cell undergoing SIGRUNB. Arrowhead points to a GFP-nucleolin-containing nuclear bubble. Indicated time points (min:s) are expressed as described in . t = 00:00 corresponds to the image captured 50 min into the recording. (B) SIGRUNB occurs before loss of plasma-membrane integrity. PI was added to the culture medium 17 h after cisplatin treatment. The still images shown were taken from Vid. S3 and are from the same field visualized separately for detection of GFP-nucleolin (upper panel) and PI (lower panel). The images show a GFP-nucleolin-expressing cell which developed nuclear bubble (arrowhead) that burst shortly thereafter. No PI staining was observed in this cell at any of the time points shown. The position of the cell nucleus undergoing SIGRUNB is shown by dashed oval and is also superimposed on the PI images. Arrows indicate PI stained apoptotic nuclei of neighboring GFP-nucleolin-negative cells (the position of those cells is shown both in the PI panel and also when superimposed on the GFP-nucleolin images). Indicated time points (min:s) are expressed as describe in . t = 00:00 corresponds to the image captured at the beginning of the recording. The images shown are from representative experiments from 8 and 3 independent experiments for A and B respectively. Bar = 10 μm.
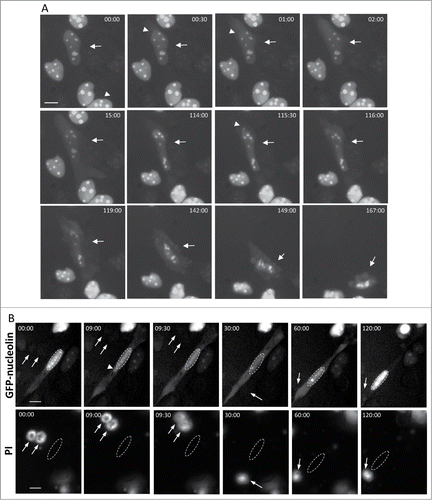
Figure 3. Bax/Bak are needed for SIGRUNB. GN-DKO MEFs were treated with 25 μM cisplatin (Cis) or 1 μM camptothecin (Camp) or left untreated. After 18 h, Hoechst 33342 was added to the culture medium and GFP-nucleolin and Hoechst fluorescence were monitored as described in . The results shown (still images from time lapse imaging) are from the same field visualized separately for detection of GFP-nucleolin (upper panels) and Hoechst 33342 (lower panels). Indicated time points (min:s) are expressed relative to the first image selected for presentation (t = 00:00) which corresponds to the image captured at the beginning of the recording. Bar = 10 μm. The images shown are from representative experiments from 2, 3 and 2 independent experiments for untreated, Cis and Camp treated cells, respectively.
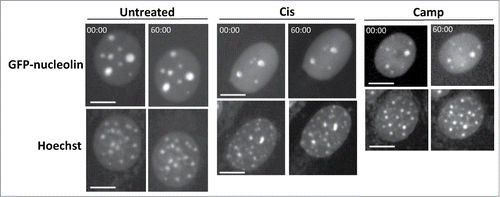
Figure 4. For figure legend see page 533.Figure 4 (See previous page). Restoration of Bax expression in Bax/Bak DKO MEFs promotes SIGRUNB in a caspase- and Bcl-xL-independent manner. Bax/Bak DKO MEFs were co-transfected with RFP-nucleolin and pEGFP or with RFP-nucleolin and GFP-Bax in the absence (A) or presence (B) of 20 μM Q-VD-OPH (QVD) or together with Bcl-xL (C). After 24 h, Hoechst 33342 was added to the culture medium and RFP-nucleolin, GFP-Bax and Hoechst fluorescence-expressing cells were monitored by live-cell time-lapse microscopy at 30-s intervals. Selected (still images from time lapse imaging) from each experiment are shown from the same field visualized separately for detection of RFP-nucleolin, GFP-Bax and Hoechst (A and C are from Vid. S4 and S5). Note that the bubbles (arrows) do not contain DNA. The dashed oval in (A) indicates the position of a DNA-lacking nuclear bubble (no Hoechst staining). Indicated time points (min:s) are expressed relative to the first image selected for presentation (t = 00:00) which corresponds to the image captured 44, 4 or 0 min into the recording (for A, B and C, respectively). Bar = 10 μm. (D) Quantification of the percentage of cells exhibiting nuclear bubbles (bubble), nuclear bubbles that ruptured (ruptured bubble) or that did not exhibit bubbles (no bubble) during recording. The number (n) of cells that were monitored for each treatment is indicated. Fisher's exact test of percentage of bubbles (ruptured bubble and bubble) revealed a significant difference (**p = 0.001) between GFP-Bax and pEGFP. The results shown are from 2, 3, 2, and 3 independent experiments for pEGFP, GFP-Bax, GFP-Bax+QVD and GFP-Bax+ Bcl-xL treatments respectively.
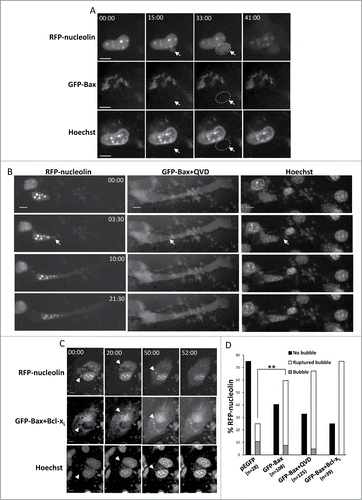
Figure 5. Identification of the Bax domains needed for promoting SIGRUNB. Bax/Bak DKO MEFs were co-transfected with RFP-nucleolin together with pEGFP, GFP-Bax or the indicated GFP-Bax mutants in the presence of 20 μM Q-VD-OPH. The cells were fixed 24 h after transfection and stained with Hoechst 33258 dye (to detect the nuclei), and GFP- and RFP-expressing cells were visualized by fluorescence microscopy. The number of cells exhibiting RFP-nucleolin only in the nuclei (nuclear), bubbles or cytosol was determined microscopically. The results are expressed as percentage of cells exhibiting nuclear, bubble or cytosolic RFP-nucleolin out of total RFP-expressing cells (n = 500 cells; 5 independent experiments). Pearson Chi-square test revealed significant differences (P < 0.0001) in the percentages of cells showing nuclear, bubble and cytosolic distribution of RFP-nucleolin between pEGFP and GFP-Bax and between GFP-Bax and each of the Bax mutants. Fisher's exact test of percentage of bubbles revealed significant differences (*P < 0.05) between GFP-Bax and GFP-Bax ΔIGDE, GFP-Bax Δα5/6 and GFP-Bax 63-65A.
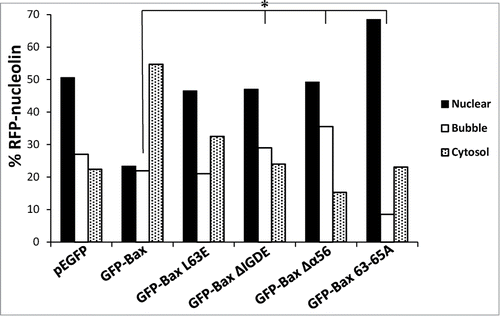
Figure 6. Bax KASH induces generation and rupture of nuclear bubbles, which are inhibited by the L63E and Δα5/6 mutations. (A) FLAG-Bax KASH is associated with the NE. Bax/Bak DKO MEFs were transfected with FLAG-Bax KASH and 24 h later were stained with anti-FLAG antibody, Mab414 antibody (to detect NPC) and Hoechst 33258 dye (to detect the nuclei). Thereafter, the cells were visualized by confocal fluorescence microscopy. Presented results are from a representative experiment (out of at least 3 independent experiments). Bar = 10 μm. (B) Bax/Bak DKO MEFs were co-transfected with RFP-nucleolin together with GFP-mini-nesprin-2G (mini-nesp2G) or FLAG-Bax KASH. Hoechst 33342 was added to the culture medium 24 h later, and RFP-nucleolin and Hoechst fluorescence was monitored by time-lapse microscopy. The presented results are quantification of the percentage of cells exhibiting bubbles, bubbles that ruptured (ruptured bubble), or no bubbles (no bubble) during the recording period (30-s intervals) out of all recorded RFP-nucleolin-expressing cells. Fisher's exact test of percentage of bubbles (ruptured bubble and bubble) revealed significant differences (**p = 0.0003) between GFP-mini-nesprin-2G and FLAG-Bax KASH. (C) Bax/Bak DKO MEFs were co-transfected with RFP-nucleolin together with GFP-mini-nesprin-2G (mini-nesp2G), FLAG-Bax KASH or the indicated FLAG-Bax KASH mutants. The cells were fixed and stained with Hoechst 33258 dye and anti-FLAG (to detect Bax KASH-and Bax KASH mutant-expressing cells) 24h later. RFP-nucleolin distribution in the nucleus, bubbles and cytosol in the transfected cells was determined microscopically. The results are expressed as the percentage of RFP-nucleolin in the nuclei, bubbles and cytosol for each treatment out of the respective RFP-nucleolin-expressing cells (n = 500 cells; 5 independent experiments). Pearson Chi-square test revealed significant differences (p < 0.0001) in the percentage of nuclear, bubble and cytosolic distribution of RFP-nucleolin between GFP-mini-nesprin-2G and FLAG-Bax KASH, or between FLAG-Bax KASH and each of FLAG-Bax KASH mutants.
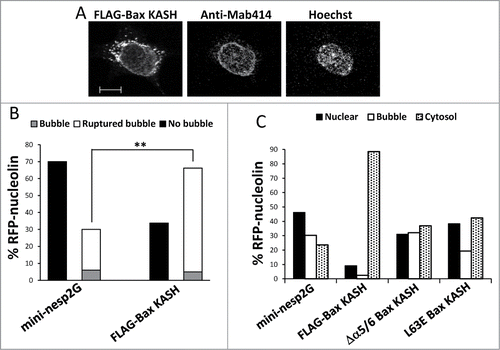
Figure 7. Stress-induced nuclear bubbles are encapsulated by impaired NE. Bax/Bak DKO MEFs were co-transfected with the expression vectors corresponding to FLAG-Bax together with RFP-nucleolin or GFP-nucleolin alone (for assessing endogenous NE proteins) and each of the indicated fluorescence versions of NE-associated proteins (for assessing nuclear bubble encapsulation) in the presence of 20 μM Q-VD-OPH. The cells were fixed and stained with Hoechst 33258 dye (to detect the nuclei) 24 h after transfection. The endogenous proteins were detected by their corresponding antibodies. Mab414 antibody was used to detect NPCs. The images of the endogenous proteins (A) were detected by confocal fluorescence microscopy and those of the overexpressed proteins (B) by epi-fluorescence microscopy. The images in each row represent the same field visualized separately for detection of RFP/GFP-nucleolin, the indicated NE protein and Hoechst-stained nuclei. Higher magnifications of the dashed boxed areas in the NE protein panel, which contain the bubble-budding sites, are shown in the right panel (B). Arrows indicate the position of the bubbles. Presented results are from representative experiments (each, out of at least 3 independent experiments). Bar = 20 μm or 10 μm for the low and high magnifications, respectively.
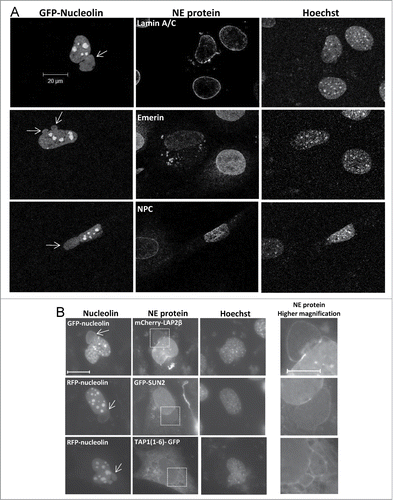
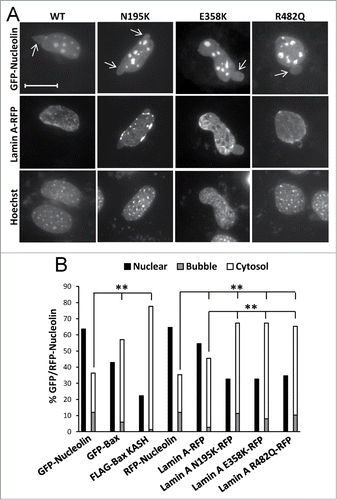
Figure 8. The effect of lamin A variants on SIGRUNB/NPR. Bax/Bak DKO MEFs were co-transfected with lamin A-RFP (WT) or the indicated lamin A mutants together with GFP-nucleolin. The cells were fixed 24 h later, stained with Hoechst 33258 dye and the distribution of lamin A variants in bubble-containing GFP/RFP-expressing cells were visualized by epi-fluorescence microscopy (A). Bar = 20 μm. To detect SIGRUNB/NPR (B) Bax/Bak DKO MEFs were transfected with RFP-nucleolin or co-transfected with RFP-nucleolin together with GFP-Bax, FLAG-Bax KASH or the indicated lamin A-RFP variants. The cells were fixed 24 h after transfection, stained with Hoechst 33258 dye and RFP-expressing cells were visualized by fluorescence microscopy. GFP/RFP-nucleolin distribution in the nucleus, bubbles and cytosol in the transfected cells was determined microscopically. The results are expressed as the percentage of GFP/RFP-nucleolin in the nuclei, bubbles and cytosol for each transfection out of the respective GFP/RFP-nucleolin-expressing cells (n = 300 cells; 3 independent experiments). Fisher's exact test of percentage of SIGRUNB/NPR (cells exhibiting GFP/RFP-nucleolin in bubbles and cells exhibiting cytosolic GFP/RFP-nucleolin) revealed significant differences (**p = 0.0001) between GFP-nucleolin or RFP-nucleolin to all other treatments; between lamin A-RFP and all the lamin A mutants examined.