Figures & data
Figure 1. Characterization of lentivectors co-expressing an array of cytokines and a PD-L1-targeted shRNA. (A) Lentivector system used to co-express cytokine genes, a PD-L1-targeted microRNA (p1), and green fluorescent protein (GFP). The histograms show PD-L1 expression in B16F0 cells (B16, left) transduced with the lentivectors co-delivering GFP-p1, and treated with IFNγ and bone marrow-derived dendritic cells (BM-DCs, right) transduced with GFP-p1 or only GFP and treated with lipopolysaccharide (LPS). Percentages and mean fluorescent intensities (MFI) for the indicated treatments are shown. Horizontal lines in the histograms represent the gate excluding 95% of non-transduced (GFP−) cells. LTR, long-terminal repeat; SFFVp, spleen focus-forming virus promoter; UBIp, ubiquitin promoter; SIN, self-inactivating LTR. (B) Flow cytometry density-plots showing cytokine expression (detected by intracellular staining with cytokine-specific antibodies) in 293T cells transduced with the indicated lentivectors. Percentages of cytokine-expressing cells are shown within the graphs. Horizontal lines represent exclusion of 95% of non-transduced cells. (C) IL15 expression assessed by inmunoblot of protein prepared from 293T cells transduced with a lentivector encoding IL15. GolgiPlug was added (top) to allow cytokine accumulation prior to cell harvest. UT, untransduced. A bioassay using SMAD-GFP cellsCitation19 was used for TGFβ detection (5.1 ± 1.03 μg TGF-β/mL lentivector stock).
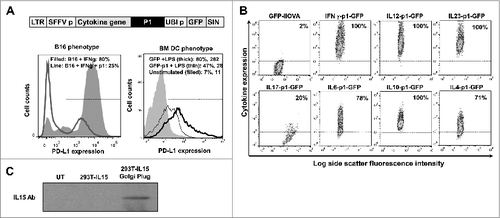
Figure 2. T-cell responses in healthy mice immunized with candidate lentivector vaccines. (A) Lentivector constructs used for mouse vaccination, comprising a cytokine from an array of cytokine genes (as indicated) co-expressed with the PD-L1 silencing microRNA (p1) and the model antigen IiOVA. The immunoblot shows IiOVA [detected using a haemagglutinin (HA) tag] and GADPH (loading control) expression in 293T cells transduced with the indicated lentivector constructs (top of the blot). LTR; long terminal repeat, SFFVp; spleen focus forming virus promoter, UBIp; ubiquitin promoter, SIN; self-inactivating LTR. (B) Percentage of OVA-specific IFNγ+ CD8+ T cells (upper left), IL17+ CD8+ T cells (upper right), granzyme B+ IFN−γ+ CD8+ T cells (bottom left), or Foxp3+ CD4+ regulatory T cells (Tregs; bottom right) among splenocytes of mice vaccinated with the indicated lentivector cytokine-p1-IiOVA constructs. Bar charts showing the mean ± S.D. (n = 5 mice per group) of cytofluorimetric analysis of splenocytes immunostained with the indicated antibodies. One representative experiment out of 2 is shown. Relevant statistical comparisons are indicated within the graph, performed by one-way ANOVA, 5 mice per group, 3 replicates; *, P < 0.05. NP, no peptide restimulation; GrzB, granzyme B. The horizontal dotted lines indicate the T-cell levels in mice vaccinated with the GFP-IiOVA lentivector (positive control), and restimulated with OVA peptides. (C) The 2 dot-plots on the left show Foxp3 expression in CD4+ CD25 OT-II transgenic T cells co-cultured for 2 days with immature dendritic cells (DCs) transduced with the lentivectors as indicated; the percentages of Foxp3+ Tregs are indicated. Bar chart (right) indicates inducible Foxp3+ Treg percentages showing the mean ± S.D. (n = 3). Relevant statistical comparisons are indicated within the graph, performed by Student's t-test; the experiment (initially performed in duplicates) was performed in triplicate with similar results achieved.
![Figure 2. T-cell responses in healthy mice immunized with candidate lentivector vaccines. (A) Lentivector constructs used for mouse vaccination, comprising a cytokine from an array of cytokine genes (as indicated) co-expressed with the PD-L1 silencing microRNA (p1) and the model antigen IiOVA. The immunoblot shows IiOVA [detected using a haemagglutinin (HA) tag] and GADPH (loading control) expression in 293T cells transduced with the indicated lentivector constructs (top of the blot). LTR; long terminal repeat, SFFVp; spleen focus forming virus promoter, UBIp; ubiquitin promoter, SIN; self-inactivating LTR. (B) Percentage of OVA-specific IFNγ+ CD8+ T cells (upper left), IL17+ CD8+ T cells (upper right), granzyme B+ IFN−γ+ CD8+ T cells (bottom left), or Foxp3+ CD4+ regulatory T cells (Tregs; bottom right) among splenocytes of mice vaccinated with the indicated lentivector cytokine-p1-IiOVA constructs. Bar charts showing the mean ± S.D. (n = 5 mice per group) of cytofluorimetric analysis of splenocytes immunostained with the indicated antibodies. One representative experiment out of 2 is shown. Relevant statistical comparisons are indicated within the graph, performed by one-way ANOVA, 5 mice per group, 3 replicates; *, P < 0.05. NP, no peptide restimulation; GrzB, granzyme B. The horizontal dotted lines indicate the T-cell levels in mice vaccinated with the GFP-IiOVA lentivector (positive control), and restimulated with OVA peptides. (C) The 2 dot-plots on the left show Foxp3 expression in CD4+ CD25 OT-II transgenic T cells co-cultured for 2 days with immature dendritic cells (DCs) transduced with the lentivectors as indicated; the percentages of Foxp3+ Tregs are indicated. Bar chart (right) indicates inducible Foxp3+ Treg percentages showing the mean ± S.D. (n = 3). Relevant statistical comparisons are indicated within the graph, performed by Student's t-test; the experiment (initially performed in duplicates) was performed in triplicate with similar results achieved.](/cms/asset/1c0697e4-ba7a-4558-a645-ea3f3c6c98bb/koni_a_945378_f0002_b.gif)
Figure 3. Local delivery of a PD-L1-targeted shRNA particularly to immune cells amplifies T-cell responses. (A) GFP-IiOVA lentivectors lacking or containing the miR-142-3p target sequence (as indicated) downstream of the IiOVA gene. Immunoblot shows IiOVA expression detected with an HA (haemagglutinin tag) antibody in 293T and bone marrow-derived dendritic cells (BM-DCs) transduced with the indicated lentivectors. Right; OVA-specific CD8+ and CD4+ responses quantified as a percentage of splenocytes from lentivector immunized mice (n=10 per group) as measured by IFNγ ELISPOT; bars indicate the mean ± S.D. Statistical comparisons were performed by one-way ANOVA; experiments performed in triplicates with similar results achieved. (B) Top, lentivectors containing the miR-142-3p targeting sequence in a IL12-p1-IiOVA backbone, with (p1) or without (Δp1) PD-L1 shRNA. Mice (n = 5 per group) were subcutaneously vaccinated and CD8+ T cell responses evaluated after OVA peptide stimulation 2-weeks later. The left graph shows the fraction of OVA-specific T cells within the CD8+ T cell population, while the right graph shows total numbers of OVA-specific CD8+ T cells among splenocytes; absence (−p) or presence (+p) of OVA peptide stimulation. LTR, long-terminal repeat; SFFVp, spleen focus-forming promoter; UBIp, ubiquitin promoter; SIN, self-inactivating LTR. Relevant statistical comparisons are shown within the graphs, calculated by one-way ANOVA; experiment performed in duplicate with similar results achieved; * P < 0.05, ** P < 0.01, *** P < 0.001.
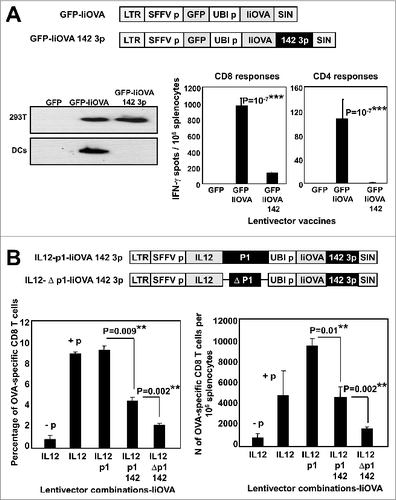
Figure 4. Lentivector vaccination delays melanoma growth in a B16 model expressing a surrogate tumor antigen. (A–C) C57Bl/6 mice (n = 5 per group) were subcutaneously vaccinated at the base of the tail with 1 × 107 lentiviral particles (as indicated) followed by subcutaneous transfer of 2 × 106 B16-IiOVA cells 2-weeks later. (A) Above, IiOVA (detected by HA-tag) and GADPH (loading control) expression assessed by immunoblot in B16 versus B16-liOVA cell lines. Below, left, tumor sizes on day 18 after B16-IiOVA subcutaneous transfer, when the first control mouse was sacrificed. Below right, time of death after B16-IiOVA transfer. Means ± S.D. are also indicated for each group as intervals. Unv, unvaccinated. (B) Immunoblots of IiOVA and GADPH expression in samples from escaping tumors in each mouse (T1 to T5) vaccinated with the indicated lentivector vaccines (shown on top) and challenged with B16-IiOVA cells. Protein extracts from B16-IiOVA cells and a tumor from unvaccinated, challenged mice (Unv T1) were used as positive controls for IiOVA expression. (C) Expression of the indicated tumor-associated antigens (TAAs) by immunoblot using specific antibodies, from escaping tumors arising in unvaccinated or IL12-p1-IiOVA-vaccinated mice challenged with B16-IiOVA cells. Each tumor was analyzed separately (T1 to T5). Ab, antibody. Statistical comparisons were performed using the non-parametric Kruskal-Wallis test; experiment performed in duplicate with similar results achieved; * P < 0.05, ** P < 0.01, *** P < 0.001.
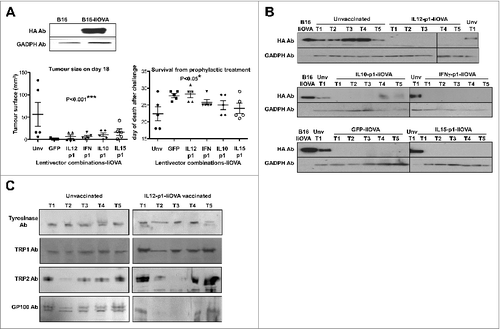
Figure 5. Prophylactic vaccination with IL12-p1-IiTrp1 enhances survival in a xenoantigen-free syngeneic B16F0 melanoma model. (A–E) C57Bl/6 mice (n = 5 mice/group) were vaccinated subcutaneously in the base of the tail with 1 × 107 lentiviral particles (as indicated) followed by B16F0 challenge 2-weeks later. (A) Top, IiTrp1 detection by immunoblot using an HA-specific antibody in 293T cells transduced with the indicated lentivectors. IiTrp1 expression levels are noticeably different (arrow). Below, tumor growth in mice vaccinated with the indicated lentivectors on top, followed by transfer of 5 × 105 B16F0 cells per mouse. (B) Top graph, Kaplan-Meier survival plot from (A) but including the indicated lentivector vaccination groups. The graphs below represent tumor size (left) on day 20, and time of death after B16F0 challenge (right). Lentivector vaccines are indicated below each graph. The arrow indicates a long-term protected mouse. C. Tumor size in mice vaccinated with the indicated lentivectors on day 13 after inoculation with B16F0 cells (2 × 106 cells per mouse). (D) Same as in C, but representing the time of death after B16F0 challenge. (E) Quantification of the percentage of TRP1-specific CD4+ and CD8+ T cells in splenocytes from mice vaccinated with the indicated lentivectors. The graphs represent IFNγ-expressing T cells, incubated overnight with dendritic cells (DCs) co-expressing GFP-IiTrp1. Data are presented as the mean ± S.D. CD8+ and CD4+ T-cell responses are represented in the left and right graphs, respectively. Unv, unvaccinated mice; Ab, antibody. Tumor sizes and survival were compared with the non-parametric Kruskal-Wallis test; experiment performed in triplicate with similar results achieved. Expansion of CD4+ and CD8+ T-cell responses between vaccinated groups were analysed by one-way ANOVA; * P < 0.05, ** P < 0.01, *** P < 0.001.
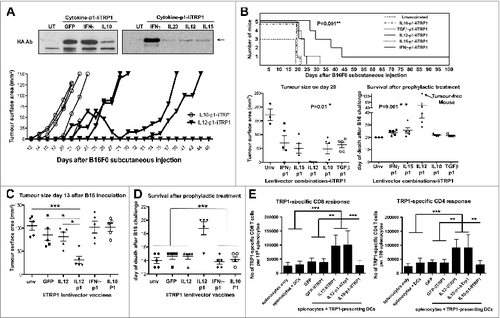
Figure 6. Therapeutic intratumor vaccination with IL12-p1-IiTrp1 can control tumor growth in a xenoantigen-free syngeneic B16F0 melanoma model. (A–C) C57Bl/6 mice were subcutaneously injected with 3 × 105 B16F0 cells/mouse (n = 5 per group) at the base of the tail. Mice were vaccinated 12 days later (average tumor size between 50–200 mm3) with 1 × 107 lentivector transducing particles intratumorally injected and tumor size was monitored every 2 days. (A) Kaplan-Meier survival plot. Pooled data from 2 independent experiments are plotted. (B) Tumor volume in mice on the day of vaccine injection (day 12) and on day 17. Data are presented as the mean ± S.D. (C) The time of death after lentivector vaccination. In this case, data from 2 independent experiments were pooled. Data are presented as the mean ± S.D. Tumor sizes and survival between groups were analysed statistically with the non-parametric Kruskal-Wallis test; duplicate experiments were performed with similar results achieved; * P < 0.05.
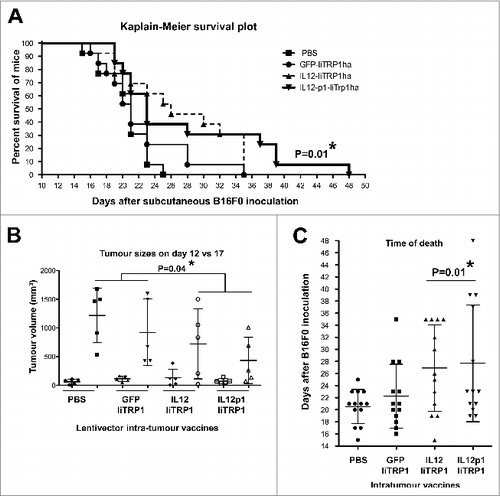
Figure 7. Delivery of PD-L1 silencing microRNA exclusively to B16F0 melanoma cells delays tumor growth. (A and B) To specifically analyse the effects of vaccination on cancer cell growth, B16F0 cells were transduced ex vivo and then injected into recipient mice (n = 5 mice per group). (A.) Top, tumor growth after subcutaneous transfer of B16F0 cells transduced with GFP-IiOVA (solid symbols) or IL12-p1-IiOVA (open symbols) lentivectors, as indicated. Below, the same as top, but with individual tumor volumes plotted at the indicated time points; bars indicate the mean ± S.D. Pooled data from 2 control experiments are included. (B) Left, tumor growth induced by B16F0 cells previously transduced ex vivo with the lentivectors GFP-IiOVA (solid symbols) and GFP-p1-IiOVA (open symbols) and subcutaneously injected into mice 4 days later. On the right, the same individual tumor volumes plotted at the indicated time points; bars indicate the mean ± S.D. Relevant statistical comparisons are shown within the graphs. Tumor sizes and survival between groups were compared with the non-parametric Kruskal-Wallis test; duplicate experiments were performed with similar results achieved; * P < 0.05, ** P < 0.01, *** P < 0.001.
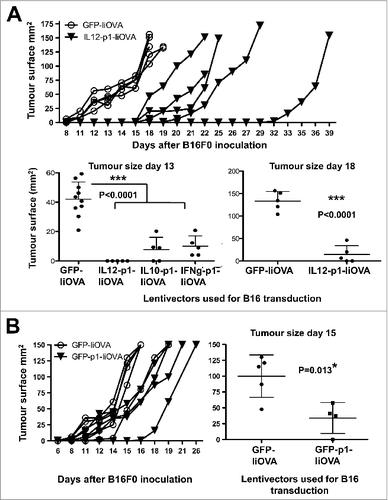
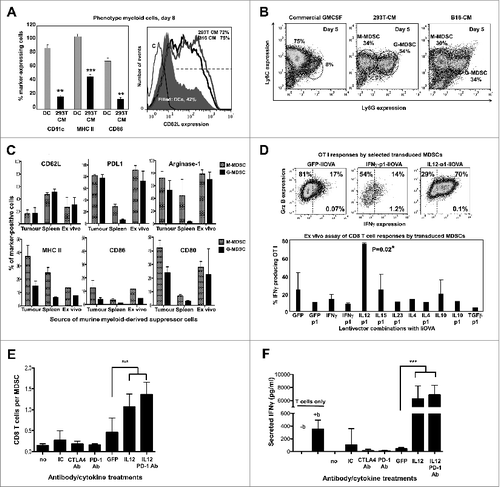
Figure 8. IL12-based lentivector vaccines exclusively strongly counteract B16F0 melanoma-infiltrating MDSC suppression of T-cell activities. (A–E) Characterization of IL-12-based lentiviral vaccines modulation of myeloid-derived suppressor cell (MDSCs) immunosuppressive activities in vitro. A. Characterization of bone marrow-derived myeloid cells cultured in the presence of conditioning media from GM-CSF expressing 293T or dendritic cells (DCs) to phenocopy the tumor microenvironment and generate MDSCs. Bar graph on the left represents the percentage of expression of the indicated markers (bottom) after 8 days in culture with DC medium or 293T-MDSC conditioning medium (CM) as indicated. Statistical comparisons between DC and MDSC markers (n = 5) are shown, analysed using a parametric t-test; more than 10 independent experimental replicates performed with similar results achieved. Histogram on the right shows CD62L expression on conventional DCs, 293T-MDSCs, and B16-MDSCs. (B) Ly6C-Ly6G density plot profiles from conventional DCs, or MDSCs differentiated in 293T- or B16-CM as indicated. Monocyte-(M) or granulocytic-(G) MDSCs are gated within the graphs with their corresponding percentages. M-MDSCs and G-MDSCs, monocytic and granulocytic MDSCs. (C) Phenotype profiling of M-MDSC and G-MDSC populations from different sources (tumor-infiltrating MDSCs, spleen MDSCs from tumor-bearing mice, and ex vivo-produced B16-MDSCs) are shown; Data are presented as the mean ± S.D. with the percentage of expression of the indicated markers on top of each graph. (D) Mixed leukocyte reaction to measure the effect of lentivector vaccine on MDSC immunosuppressive activities. MDSCs transduced with the indicated lentivector were co-cultured with OVA-specific OT-I CD8+ T cells at a ratio of 1:10 (MDSC:T cells). Top flow cytometry density plots show IFNγ/Granzyme B (Grz B) expression profiles from OT-I cells. The bottom graph represents the percentage of IFNγ-producing OT-I cells in the MDSC-T cell assay. Statistical comparisons between groups (n = 5 per group) were performed with the parametric test one-way ANOVA; the experiment was repeated numerous times with similar results achieved. (E) T cell proliferation assay. Figure 8 (See opposite page). Number of CD8+ T cells in standard suppression assays using B16-MDSCs, represented as a bar graph. CD8+ T cells were activated with anti-CD28 anti-CD3 beads. Anti-PD1 antibody, anti-CTLA-4 antibody, and 250 pg IL12 were added as indicated. GFP indicates the addition of control supernatants (non-antibody control) from 293T cells expressing GFP. Statistical comparisons between groups (n = 3 per group) were performed with the parametric test one-way ANOVA; the experiment was repeated independently twice with similar results achieved. (F) The same as in e but plotting the amount of secreted IFNγ after 72 hours of co-culture, as assessed by ELISA. no, no treatment; IC, isotype control; b, anti-CD3/CD28 activation beads. GFP indicates the addition of supernatant from 293T-expressing GFP. Statistical comparisons between groups (n = 3 per group) were performed with the parametric test one-way ANOVA; the experiment was repeated independently twice with similar results achieved; * P < 0.05, ** P < 0.01, *** P < 0.001; data are presented as the mean ± S.D.