Figures & data
Figure 1. Intradermal administration of apoptotic blebs or apoptotic cell remnants does not affect the number and subset distribution of migratory DC. (A–C) Analysis of skin emigrated dendritic cells (DCs) following injection of medium, apoptotic cell remnants (ACR) or apoptotic blebs. Cultured human skin explants were intradermally injected with leukemic cell-derived blebs or ACR either alone (in plain medium) or co-administered with of IL-4/GM-CSF. A. DCs that egressed were harvested and counted after 48 hours post injection: Medium (−/−), n = 4; IL-4/GM-CSF, n = 9. (B) Egressed DCs were harvested and immunostained for CD11c, CD1a and CD14 and analyzed via fluorescence cytometry in order to discriminate LCs and the 4 dermal DC subsets, as indicated. (C) Skin DC subset distribution was analyzed after injecting either medium alone (grey bars), ACR (black bars), or blebs (white bars) in plain medium (left graph; n = 5), or supplemented with IL-4/GM-CSF (right graph; n = 5). Shown are the mean values ± SEM. Statistical significance was determined by one-way Anova.
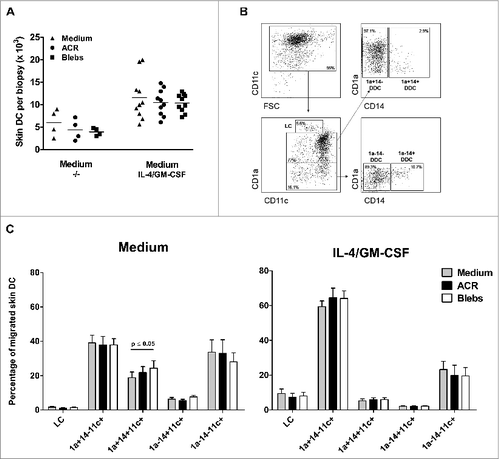
Figure 2. Quantification of the in situ uptake of apoptotic material by skin-emigrated dendritic cells. (A–E) Carboxyfluorescein succinimidyl ester (CFSE)-labeled apoptotic cell remnants (ACR) or blebs were injected into human skin explants, and the dendritic cell (DC) uptake was quantified using immunostaining and fluorescence cytometry. (A) Viable CD11c positive DCs were plotted against CD1a to discriminate Langerhans cells (LCs) as CD1ahi/CD11cdim. CD1a+ and CD1a− DCs were plotted against CD14 for the discrimination of the various dermal dendritic cell (DDC) subsets –as indicated. (B–E) For the analysis of the in situ uptake of CFSE-labeled apoptotic material, CFSE positivity of the egressed skin DC was determined by flow cytometry (indicated by percentages positive cells per subset; representative example from n = 9 shown). Quantification of the ingestion of apoptotic cell remnants (ACR, black bars) or blebs (white bars) in situ, is either displayed as the CFSE positive skin DC as a percentage of the total CD11c+ egressed skin DC (B and D) or as a percentage of CFSE positive cells within a subset (C and E). The apoptotic cell fractions were administered either in plain medium (B and C, n = 6) or with IL-4 and GM-CSF (D and E, n = 9). Shown are the mean values ±SEM. Statistical significance was determined by 2-tailed Student's t-test.
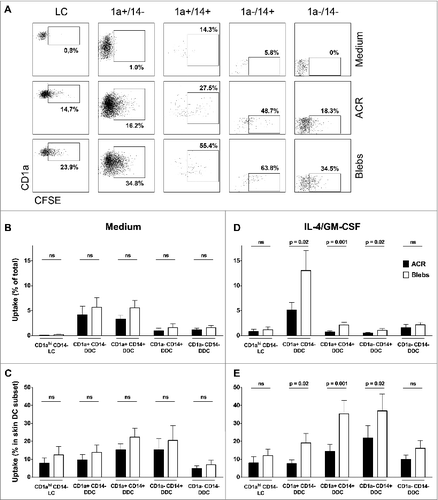
Figure 3. Effect of intradermally injected apoptotic remnants or blebs on maturation state of DC subsets subsequently emigrated from skin. Expression of the maturation markers CD83 and CD86 on the different DC subsets, that egressed from the skin explants after intradermal injection of carboxyfluorescein succinimidyl ester (CFSE)-labeled apoptotic cell remnants (ACR) or blebs co-injected with IL-4/GM-CSF versus media. Expression levels of the differentiation markers were determined by immunostaining and fluorescence cytometry. CD83 (top row) and CD86 expression is shown on DC which had ingested apoptotic material (black bars), and DC that had not taken up either ACR or blebs (white bars), as compared to background levels after injecting medium alone (grey bars). Shown are the mean values ± SEM (n = 9). Statistical significance was determined by 2-tailed Student's t-test; * P-value < 0.05; ** P-value < 0.01; *** P-value < 0.001.
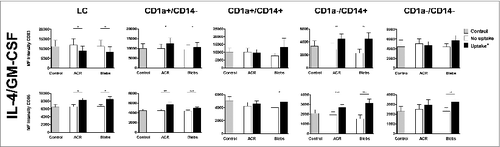
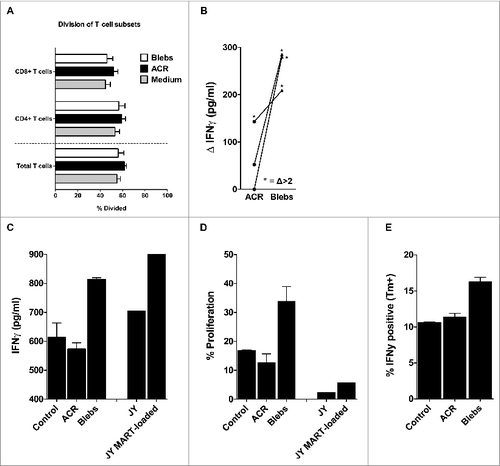
Figure 4. Mixed leukocyte reaction and antigen cross-presentation by egressed skin DCs after intradermal delivery of apoptotic cell remnants or blebs. (A) After 48–72 hours following intradermal injection of the indicated apoptotic cell fractions, the egressed dendritic cells (DCs) were harvested and co-cultured for 6 days with carboxyfluorescein succinimidyl ester (CFSE)-labeled allogeneic peripheral blood lymphocytes (PBLs) from healthy donors, in an allogeneic mixed leukocyte reaction (MLR) at a 1:10 ratio of DCs:PBLs. Shown are the mean percentages of divided PBLs after 6 days of co-culture, with the percentage of CFSE dilution utilized as a measure of CD4+ and CD8+ T-cell proliferation (n = 7). (B) DCs that egressed from the skin biopsies after injecting either wild-type or MART-1 expressing apoptotic cell remnants (ACRs) or blebs, were co-cultured with a MART-126-35 recognizing and HLA-A2 restricted cytotoxic T lymphocyte (CTL) line (> 95% pure) for 24 hours. Antigen-specific activation was determined by deducting the levels of interferon γ (IFNγ) produced following the injection of wild-type ACRs or blebs from the levels produced after injecting MART-1 expressing apoptotic fractions (n = 3). Antigen-specific induction of IFNγ levels exceeding those achieved by cross-presentation of wild-type ACR or blebs by more than 2-fold are indicated by asterisks. (C–D) Emigrated skin DCs stimulated by injecting either ACR or blebs derived from MART-1 expressing HL60 were co-cultured for 24 hours with a CFSE-labeled MART-1 specific cytotoxic T lymphocyte (MART-1 CTL) clone generated by stimulation with MART-1 loaded JY cells. IFNγ production was analyzed in the supernatant by cytokine bead array after 24 hours (C; n = 1) and proliferation, as measured by CFSE dilution (as in A, above), after 6 days (D; n = 1). (E) IFNγ production by a MART-1 CTL line (> 95% pure) as assessed by intracellular immunostaining and cytofluorimetric analysis, after 5 hours of co-culture with skin DCs, that had migrated from ACR- or bleb- (derived from MART-1 expressing HL60) injected skin biopsies (n = 1). Shown are the mean values ± SEM.