Figures & data
Figure 1. T-cell responses against FoxO3 in cancer patients compared to healthy individuals. (A–D) Peripheral blood mononuclear cells (PBMCs) derived from cancer patients or healthy donors were assayed for reactivity to FoxO3 peptides by interferon γ (IFNγ) ELISPOT assay. (A) T-cell responses against the FoxO392–100 (LLLEDSARV) peptide as measured by interferon γ (IFNγ) ELISPOT. PBMCs from 5 patients with renal cell carcinoma (RCC), 16 with malignant melanoma (MM), 6 breast cancer patients (CM) and 10 healthy donors (HD), were stimulated once with peptide and screened for responses against FoxO392–100 using IFNγ ELISPOT. The average number (in triplicates) of FoxO392–100-specific spots (after subtraction of spots with irrelevant HIVpol476–484 added peptide) was calculated per 5 × 105 PBMCs for each patient. A Mann-Whitney test was used to determine the significance of the response against the FoxO392–100 peptide in cancer patients compared to healthy donors; P-value = 0.0001. (B) In vitro IFNγ ELISPOT responses against the FoxO3118–126 (GLSGGTQAL) peptide measured in a similar manner as for the FoxO392–100 peptide. The average number (in triplicates) of FoxO3118–126 -specific spots (after subtraction of spots with irrelevant HIVpol476–484 peptide) were calculated per 5 × 105 PBMCs for each patient, with the exception of patient RCC44 (as indicated with *) who displayed a robust response per 2 × 105 PBMCs. A Mann-Whitney test was used to determine the significance of reactivity of FoxO3118–126 specific T cells in cancer patients compared to healthy donors; P-value = 0.0125. (C) Examples of significant ELISPOT responses to FoxO392–100 (black bars) as compared to irrelevant HIVpol476–484 peptide (gray bars) among PBMCs from different cancer patients, including 3 melanoma patients (MM03, MM25 and MM129), a breast cancer patient (CM.16) and 2 renal cell carcinoma patients (RCC41 and RCC44). (D) Examples of significant ELISPOT responses to FoxO3118–126 (black bars) as compared to irrelevant HIVpol476–484 peptide (gray bars) in PBMCs from different cancer patients, including a melanoma patient (MM129) and 2 renal cell carcinoma patients (RCC41and RCC44).
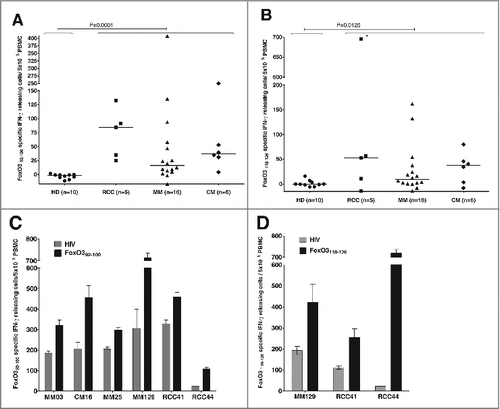
Figure 2. FoxO3118–126-specific T cells are present among PBMC ex vivo and are able to expand in vitro. (A–B) FoxO3118–126 responsive peripheral blood mononuclear cells (PBMCs) from cancer patients (refer to Figure 1) were assayed for reactivity to FoxO3 peptides without prior peptide stimulation by ELISPOT assay to detect interferon γ (IFNγ). Ninety-six-well plates coated with anti-human IFNγ antibody were incubated with PBMCs and the indicated peptide (in triplicates). (A) Ex vivo IFNγ ELISPOT as measured against either HIVpol476–484 (gray bars) or FoxO3118–126 (black bars) reactivity among PBMCs from 3 cancer patients. The renal cell carcinoma (RCC) patients RCC41 and RCC44 exhibited significant responses against FoxO3118–126 ex vivo; one-tailed Student's t-test. (B) Representative images (RCC44) from an IFNγ ELISPOT response against FoxO3118–126 compared to HIVpol476–484 ex vivo. (C) Immunofluorescence staining of PBMCs from RCC44 with fluorophore-conjugated tetramer-complexes HLA-A2/HIVpol476–484-PE and HLA-A2/HIVpol476–484-APC (left) or HLA-A2/FoxO3118–126-PE and HLA-A2/FoxO3118–126-APC (right). (D) Expansion of FoxO3118–126-specific T cells from PMBCs from RCC44 by culturing ex vivo, as measured by immunofluorescence staining with tetramer-complexes and cytofluorimetric analysis. The staining of the specific T-cell culture was performed after either several rounds of in vitro peptide stimulations using HLA-A2/HIVpol476–484-PE and HLA-A2/HIVpol476–484-APC (left panel) or HLA-A2/FoxO3118–126-PE and HLA-A2/FoxO3118–126-APC (second panel), or after further purification of FoxO3-specific T cells by tetramer-based cell sorting with HLA-A2/FoxO3118–126-PE and HLA-A2/FoxO3118–126-APC (third panel), or with HLA-A2/FoxO3118–126-PE or HLA-A2/FoxO3118–126-APC (right panel).
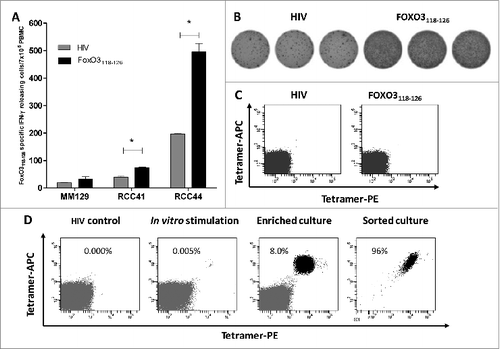
Figure 3. Cytotoxic functional capacity of cancer patient-derived FoxO3118–126-specific T cells (A–D). Tetramer-staining and cytotoxicity-testing of a sorted T-cell culture with (E) low, (F) high and (G) pure FOXO3-specific T cells. Immunofluorescence staining and cytofluorimetric analysis of ex vivo-expanded, peptide-stimulated T cells. T cells were enriched from a renal cell carcinoma patient (RCC44) by several rounds of FoxO3-peptide stimulation and culturing in the presence of cytokines. (A) Tetramer analysis of FoxO3118–126-specific T cells from RCC44 T-cell culture stained with the following fluorophore-conjugated tetramers: HLA-A2/HIVpol476–484-PE and HLA-A2/HIVpol476–484-APC (top) or HLA-A2/FoxO3118–126-PE and HLA-A2/FoxO3118–126-APC (bottom). (B) Intracellular tumor necrosis factor α (TNFα) and interferon γ (IFNγ) staining analysis of the FoxO3118–126-specific culture stimulated with either HIVpol476–484 (top) or FoxO3118–126 (bottom) peptide for 5 h. (C and D) Cell surface expression of CD107a and production of IFNγ (C) and TNFα (D) by the FoxO3118–126–specific T-cell culture stimulated with either HIVpol476–484 (top) or FoxO3118–126 (bottom) peptide for 5 h. (E) Staining (left) of the specific T-cell culture by HLA-A2/HIVpol476–484-PE and HLA-A2/HIVpol476–484-APC (top) or HLA-A2/FoxO3118–126-PE and HLA-A2/FoxO3118–126-APC (bottom) and cytotoxic killing (right) by the same T-cell culture of T2 cells pulsed with irrelevant HIV-peptide (circles) or with FoxO3118–126 (squares). (F) FoxO3-specific T-cell culture tetramer-staining and cytotoxic killing of T2 cells loaded with either irrelevant HIV-peptide (circles) or with FoxO3118–126 (squares). (G) High specificity of FoxO3-specific T-cell culture enriched by tetramer-based cell sorting. tetramer-staining and cytotoxic killing of T2 cells loaded with either irrelevant HIV-peptide (circles) or with FoxO3118–126 (squares).
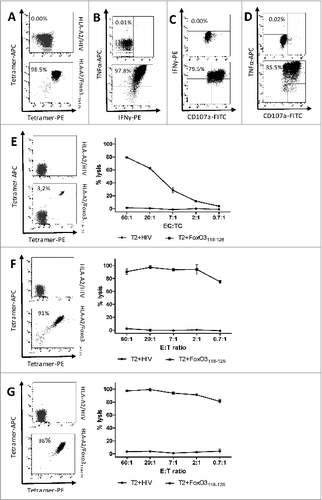
Figure 4. T-cell mediated killing is dependent on HLA-expression and intracellular levels of FOXO3. Cytotoxicity of FoxO3-specific T cells and dependence on target cell levels of HLA and FoxO3 was measured by chromium51-release assay with and without exogenous HLA or siRNA-mediated knockdown of FoxO3. (A) Lysis of the FoxO3-expressing, HLA-class I-deficient leukemic cell line K562 (circles) as well as K562 cells transduced with either HLA-A1 (K562/HLA-A1; squares) or HLA-A2 (K562/HLA-A2; stars) by FoxO3118–126-specific T cells. (B) Lysis of K562/HLA-A2 transfected with either control siRNA (circles) or FoxO3 siRNA (squares) by FoxO3118–126-specific T cells. Statistical analysis was performed by unpaired Student's t-test; * P<0 .05. (C) Expression level of FOXO3-protein examined by Western Blot analysis in the cell lines K562, K562/HLA-A1, K562/HLA-A2, K562/HLA-A2 transfected with either FoxO3 siRNA or control siRNA. Densitometric quantification and blotted membranes are shown.
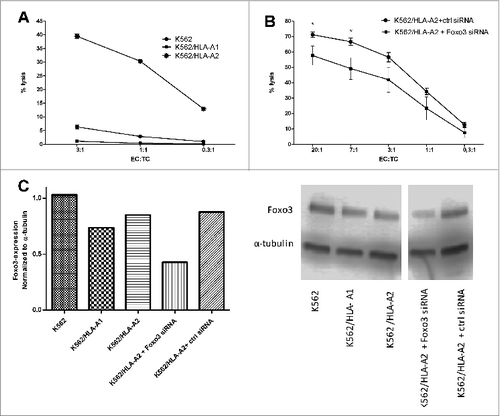
Figure 5. FoxO3118–126-specific T cells recognize and kill autologous dendritic cells. (A-D) Dendritic cells were derived from cancer patient peripheral blood monocytes enriched by adherence to plastic and cytokine stimulation ex vivo. FoxO3118–126-specific T cells response to autologous matured DCs and monocytes were determined by intracellular staining and fluorescence cytometry (A and B) or cytotoxicity assay (C). (A) Intracellular interferon γ (IFNγ) staining of FoxO3118–126-specific T cells stimulated with either directly isolated monocytes (left), or in vitro generated DC (right) for 5 h. (B) Intracellular tumor necrosis factor α (TNFα) staining of FoxO3118–126-specific T cells stimulated with either directly isolated monocytes (left), or in vitro generated DC (right) for 5 h. (C) Chromium51-release assay to determine the FoxO3118–126-specific T cell-mediated lysis of autologous, in vitro generated DCs transfected with control siRNA (circles) versus a FoxO3-specific siRNA (squares). Statistical analysis was performed by unpaired Student's t-test; *P < 0 .05. (D) Expression level of FOXO3-protein examined by Western Blot analysis in the autologous, in vitro generated DC transfected with a FoxO3-specific siRNA or autologous, in vitro generated DC (mock). Densitometric quantification and blotted membranes are shown.
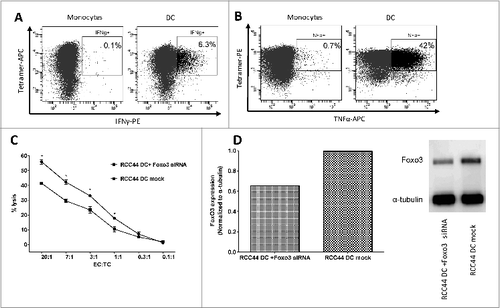
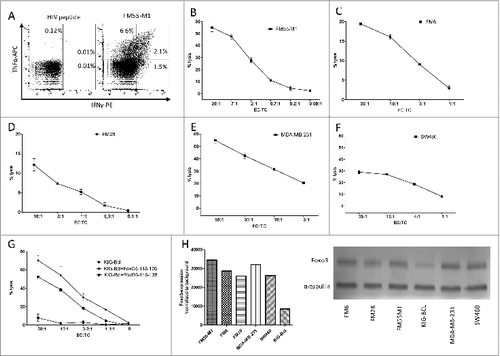
Figure 6. FoxO3118–126-specific T cells recognize and kill a broad variety of cancer cells. (A-G) Cancer patient derived FoxO3118–126-specific T cells responses to melanoma, breast and colon cancer cells, as well as an antigen presenting B cell line were determined by intracellular staining and fluorescence cytometry (A) or cytotoxicity (chromium51 release) assay (B-G). (A) Intracellular tumor necrosis factor α (TNFα) and interferon γ (IFNγ) staining analysis of the FoxO3118–126-specific T-cell culture stimulated with either HIVpol476–484 (left) or FM55-M1 cells (right) at an effector to target cell (EC:TC) ratio of 10:1 for 5 h. (B) Lysis of the HLA-A2+ melanoma cell line FM55-M1 (squares) by a FoxO3118–126-specific T-cell culture at different EC:TC ratios. (C-F) FoxO3118–126-specific cultured T cell-mediated lysis of the HLA-A2+ melanoma cell line FM6 (C), the HLA-A2+ melanoma cell line FM28 (D), the HLA-A2+ breast cancer cell line MDA-MB-231 (E), and the HLA-A2+ colon cancer cell line SW480 (F). (G) FoxO3118–126-specific cultured T cell culture-mediated lysis of the HLA-A2+ EBV transfected B-cell line (KIG-Bcl) alone (circles) versus cells pulsed with long-peptide FoxO3116–138 (stars) or the FoxO3118–126-epitope (squares). (H) Densitometric quantification and blots of the expression of FOXO3 in a Western Blot of the cancer cell lines (FM55-M1, FM6, FM28, MDA-MB-231, and SW480) and the EBV-transfected B-cell line (KIG-Bcl).