Figures & data
Table 1. Sequences of IGF-IR peptide B-cell epitopes and chimeric peptide vaccines. The amino acid sequences of insulin growth factor receptor 1 (IGF-1R) peptides as well as the epidermal growth factor receptor (EGFR/HER-1) and v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2/HER-2) peptides, and the chimeric B-cell epitope vaccines engineered with the “promiscuous” T cell epitope of the measles virus fusion protein (MVF; italics) are shown. The B-cell epitopes were all acetylated (Ac) at the N-terminus and amidated (NH2) at the C- terminus. The flexible linker sequence GPSL was used in collinearly synthesizing the vaccines with MVF is underlined in the table and the molecular weights for each inhibitor is indicated.
Figure 1. IGF-1R peptide mimics inhibit proliferation of pancreatic and breast cancer cells. The indicated cancer cells were incubated with insulin growth factor receptor 1(IGF-1R) peptide mimics and irrelevant peptide (IRR) at various concentrations (ranging from 25–150 μg/mL) and incubated for 3 d prior to the addition of the tetrazolium dye MTT. The proliferation inhibition rate was calculated using the formula (OD normal untreated- OD peptide treated / OD normal untreated) × 100. Results are an average of 2 different experiments with each treatment performed in triplicate;error bars represent the mean ± S.D.; irrelevant peptide was used as the negative control.
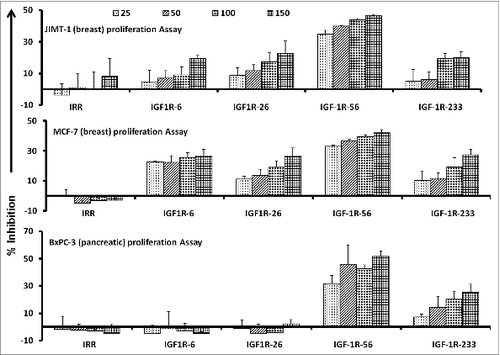
Figure 2. IGF-1R peptide vaccine is immunogenic in rabbits and generates antibodies that specifically interact with human IGF-1R. (A–C). Rabbits were immunized intramuscularly 3x per week over 3 week intervals with 1 mg of the indicated chimeric peptide vaccine emulsified with the adjuvant nor-muramyl dipeptide derivative (nor-MDP) in SEPPIC ISA 720 as the vehicle. (A) Antibody titers were defined as the reciprocal of the highest serum dilution that gives an absorbance of 0.2 after subtracting the presera levels. 1y +3w represent blood drawn 3 weeks after the first immunization while 3y +3w represent blood drawn 3 weeks after the third immunization. (B) Binding of vaccine antibodies to recombinant human IGF-1R and human cancer cell lines by ELISA analysis. Binding of purified vaccine antibodies to recombinant human IGF-1R protein was measured and specificity evaluated by dilution of the antibodies and a corresponding gradual decreased in binding. The antibodies from the presera showed no binding and were used as the negative control. In (A and B), results are an average of 2 different experiments with each treatment performed in duplicates. (C) Human cancer cell lines (JIMT-1, MCF-7 and BxPC-3) were incubated with peptide vaccine antibodies and the extent of cell binding was evaluated by immunofluorescence staining andcytofluorimetric analysis. A shift of the histogram to the right indicates increase in binding relative to pre-immune antibodies used as a negative control. Experiments were performed twice with similar results achieved and shown is a representative histogram from each treatment.
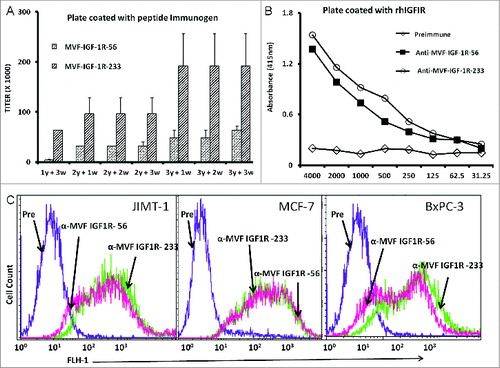
Figure 3. IGF-1R peptide mimics and vaccine antibodies are capable of eliciting cancer cell apoptosis. Apoptosis was evaluated by measuring caspase activity after treatment with 150 μg/mL peptide vaccine antibodies. Cells were plated in 96-well plates and treated with inhibitors for 24 h prior to the addition of the caspase reporter (Casp-GLO) reagent. caspase activity with a luminometer. Normal rabbit IgG was used as a negative control. Results showed that the antibodies and peptide mimics increased caspase activity, indicating induction of apoptosis. Results shown are averages of 3 different experiments with each treatment performed in duplicates and error bars represent the mean ± S.D.
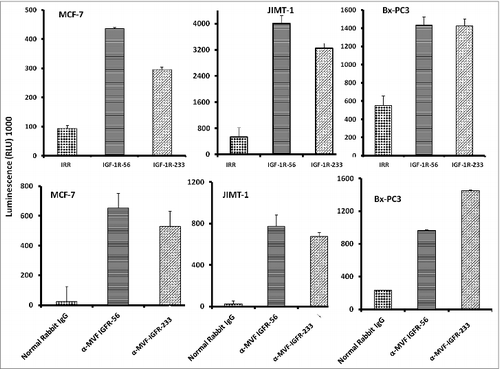
Figure 4. IGF-1R peptide mimics and vaccine antibodies Inhibits of IGF-1R phosphorylation in breast (MCF-7 and JIMT-1) and pancreatic (BxPC-3) cancer cells. The indicated cancer cells were treated with the various inhibitors or controls (see below) at a concentration of 100 μg/mL and incubated for 1 h before stimulating with 50 ng/mL IGF-1 ligand for 10 min. Treated ells were then lysed in RIPA lysis buffer, and receptor phosphorylation was measured using human phospho-IGF-1R ELISA kits from R&D Systems. Cyclolignan PPP is an IGF-1R tyrosine kinase inhibitor (TKI) and was used as a positive control. Irrelevant peptide (IRR) and normal rabbit IgG (NrIgG) were used as negative controls. Results are an average of 2 different experiments with each treatment performed in triplicates and error bars represent the mean ± S.D.
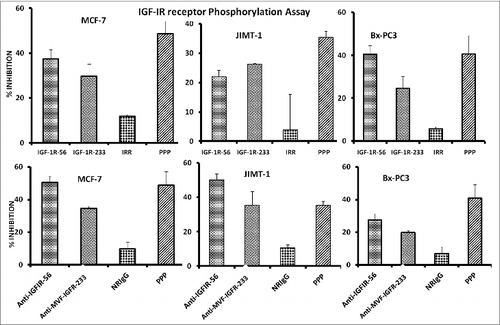
Figure 5. Vaccine antibodies induce ADCC of cancer cells. Target cells (MCF-7, JIMT-1, and BxPC-3) were plated in the presence of human peripheral blood mononuclear cells (PBMCs) at an effector to target ratio of 100:1, 20:1, and 4:1 in triplicates, incubated with 100 μg/mL of the purified anti-peptide rabbit Abs or controls, and incubated for 2–4 hours at 37°C. Antibody dependent cell cytotoxicity (ADCC) was measured by a non –radioactive assay using the aCella-TOX reagent according to manufacturer's instructions. Results are an average of 2 different experiments with each treatment performed in triplicates and error bars represent the mean ± S.D.
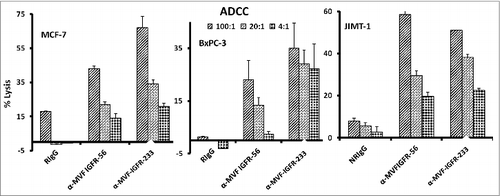
Figure 6. IGF-1R peptide mimic therapy inhibits breast and pancreatic tumor growth. Effects of IGF-1R peptide mimics in transplantable mouse models of breast (A–C) and pancreatic (D and E) cancer. Balb/c SCID host mice (n=5 per group) at the age of 5–6 weeks old were injected subcutaneously with 5 × 10 6 human cancer cells (JIMT-1 and BxPC-3). From the day of tumor challenge (day 0), the mice were treated intravenously with 200 μg of the indicated peptide mimic or 500 μg of peptide vaccine antibody weekly until week 7. The percentages of tumor weights after treatment are shown; error bars represent the mean ± S.D.
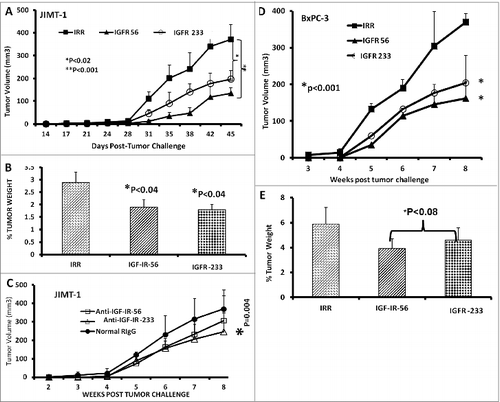
Figure 7. Combination therapy with IGF-1R and HER-1 peptide mimics inhibits cancer cell proliferation and IGF-1R receptor phosphorylation. (A) Breast (5,000/well) and pancreatic (4,000/well) cancer cells were plated in a 96-well plate, treated with a combination of epidermal growth factor receptor (EGFR,/ HER-1) and IGF-IR peptide mimics as inhibitors and subject to MTT proliferation assay. (B and C) HER-1 and IGF-1R phosphorylation was measured using human phospho-HER-1/IGF-1R ELISA kits (R&D systems) after combination peptide mimic treatment of 1 x 106 cancer cells in a 6-well plate. Each inhibitor was used at a concentration of 200 μg, which was determined after a dose-dependent titration from 12.5 μg to 200 μg. Results are an average of 2 different experiments with each treatment performed in triplicates and error bars represent the mean ± S.D.; *p < 0.001; **p < 0.005; #p < 0.03.
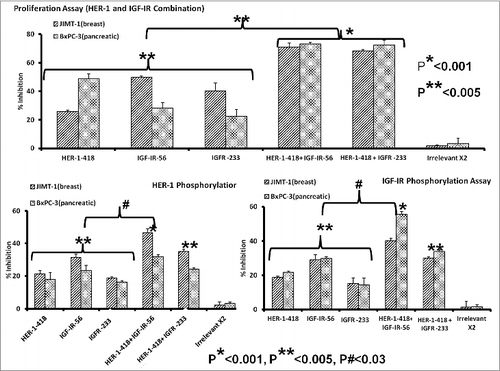
Figure 8. Induction of cancer cell apoptosis and ADCC by combination treatment with HER-1 and IGF-1R peptide vaccine antibodies. Cancer cells treated in vitro with HER-1 and IGF-1R peptide vaccine antibodies were monitored for cell death induction via apoptosis assay and antibody dependent cellular cytotoxicity (ADCC) assay. (A) Breast (JIMT-1) and pancreatic (BxPC3) cancer cells apoptosis was evaluated by measuring caspase activity after combination treatment with 100 μg/mL each HER-1 and IGF-1R peptide vaccine antibodies . Cells were plated in 96-well plates and treated with inhibitors for 24 h prior to adding Caspase-GLO reagent and read with a luminometer. (B) Target cells (JIMT-1) were incubated with 100 μg of single or combination treatments of the peptide vaccine antibodies and assayed in the presence of human peripheral blood mononuclear cells (PBMCs) at an effector to target ratio of 100:1, 20:1, or 4:1 in triplicates. Lysis was measured using the acella-TOX kit according to manufacturer's instructions. Normal rabbit IgG was used as negative controls Results are an average of 2 different experiments with each treatment performed in triplicates;error bars represent the mean ± S.D.; *p < 0.001; **p < 0.05.
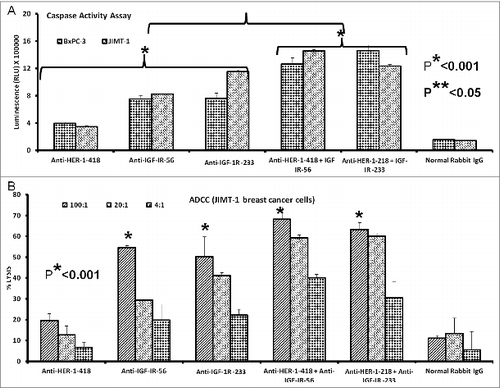
Figure 9. IGF-1R and HER2 antibody combination treatment inhibits proliferation and IGF-1R and HER-2 receptor phosphorylation in trastuzumab-resistant breast cancer cells. (A) MTT proliferation assay using combination treatment with v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2/HER-2) and IGF-1R antibodies (200 μg each) as inhibitors in JIMT-1 trastuzumab-resistant (plated at 4,000 cells/well) and BT-474 trastuzumab-sensitive (plated at 5,000 cells/well) breast cancer cells in a 96-well plate. (B and C) HER-2 and IGF-1R receptor phosphorylation was measured in 1 x 106 cancer cells per 6-well plate after combination treatment with IGF-1R and HER-2 antibodies (200 μg each) using human phospho-HER-2/IGF-1R ELISA kitsResults are an average of 2 different experiments with each treatment performed in duplicates and error bars represent the mean ± S.D.. p < 0.003, for proliferation, *p < 0.05 for HER-2 phosphorylation and **p < 0.005 for IGF-1R receptor phosphorylation.
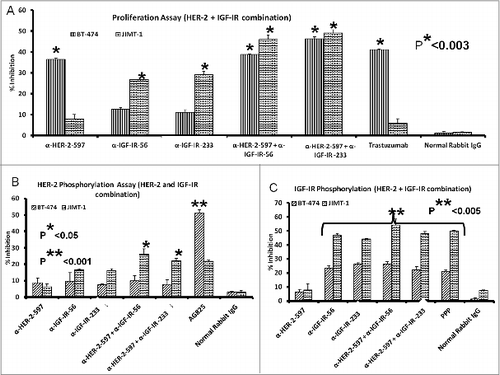
Figure 10. Combination treatment with HER-2 and IGF-1R vaccine antibodies potentiates breast cancer cell apoptosis and ADCC. Induction of apoptosis and ADCC by combination treatment with HER-2 and IGF-1R peptide vaccine antibodies. (A) Apoptosis was evaluated by measuring caspase activity after combination treatment with 100 μg/mL each with anti-HER-2 and IGF-1R peptide vaccine antibodies () in both trastuzumab-sensitive (BT-474) and resistant (JIMT-1) breast cancer cells. Cells were plated in 96-well plates and treated with inhibitors for 24 h before adding caspase (Caspase-GLO) reagent and subsequent luminescence read with a luminometer. Normal rabbit IgG was used as negative controls. (B) Combination treatment with vaccine antibodies induces ADCC of cancer cells. Target cells (JIMT-1) were incubated with 100 μg of single or combination treatments of the peptide vaccine antibodies and assayed in the presence of human peripheral blood mononuclear cells (PBMCs) at an effector to target ratio of 100:1, 20:1, and 4:1 in triplicates. Effects of combination treatment of HER-2 and IGF-1R are shown; tumor cell lysis was measured as described in . Results are an average of 2 different experiments and error bars represent the mean ± S.D.
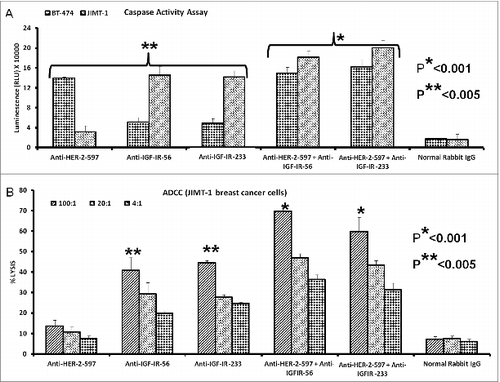
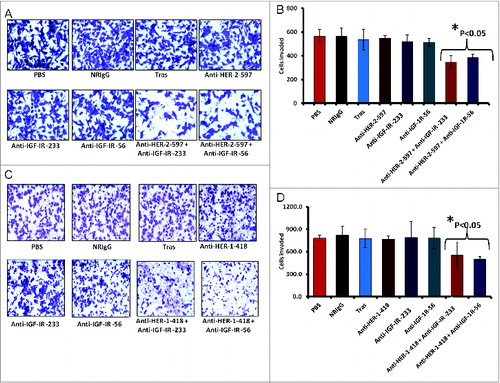
Figure 11. Co-targeting HER-2 and IGF-1R suppresses invasiveness of trastuzumab-resistant breast cancer cells. (A and B) JIMT-1 cells were pretreated for 48 h with the indicated inhibitors before being seeded in Boyden matrigel-coated chambers with 10% FBS. After 24 h, invasion was measured by taking photos and counting the number of invaded cells in 10 random fields; results represent the average of triplicates per group. Representative photos (A) and quantitation of the invasive cells under the different treatment conditions (B). (C and D) Similar inhibition of invasive capabilities of JIMT-1 cells were observed via co-targeting HER-1 and IGF-1R. Boyden chamber migration and invasion assay was performed as described above; photographs (C) and quantitative results of invasive cell number are shown in (D). Results are an average of 2 different experiments and error bars represent the mean ± S.D.. Statistical analysis demonstrated that combination treatment with HER-2 and the IGF-1R vaccine antibodies or HER-1 and IGF-1R vaccine antibodies significantly (p < 0.05) inhibited invasion.