Figures & data
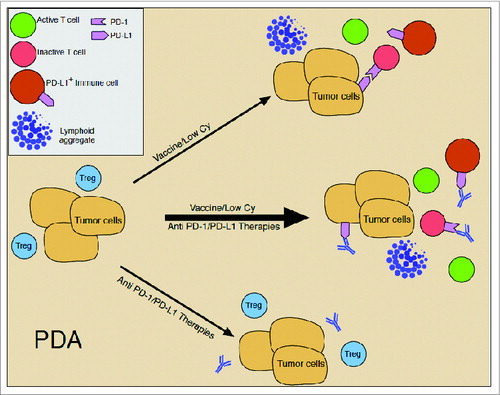
Figure 1. Model explaining the inefficacy of single agent immunotherapy for pancreatic cancer. At baseline, pancreatic tumors are predominantly infiltrated with immunosuppressive regulatory cells, such as T regulatory cells (Treg), and few effector T cells (T cell); express low levels of PD-L1 on tumor cells; and are infiltrated with few to no PD-L1-expressing innate immune cells (PD-L1+ immune cell). In this non-immunized and inactive state, treatment with immune checkpoint inhibitors alone, such as anti-PD-1/PD-L1, is hampered by the lack of effector T cells to act on. Treatment with the GVAX vaccine (Vaccine) combined with low dose cyclophosphamide (Cy) converts the pancreatic tumor microenvironment from a relatively inactive to an active state by inducing the infiltration of effector T cells and the formation of intratumoral tertiary lymphoid aggregates (Lymphoid aggregate). However, cytokines produced by the immune cells that are induced to traffick the tumor, such as IFNγ, induce the upregulation of immunosuppressive mechanisms, such as the upregulation of PD-1 and PD-L1 expression. These countering immunosuppressive mechanisms limit the activity and efficacy of vaccination, but also prime the pancreatic tumor microenvironment for immune modulators, such as checkpoint-inhibitors. Thus, optimal activity and antitumor efficacy of either of these single approaches is dependent on the other.