Figures & data
Table 1. T cell-mediated immune response of patients enrolled in the trial
Figure 1. T-cell responses to MAAs in PBMCs of melanoma patients undergoing NGR/VAX treatment. Freshly isolated peripheral blood mononuclear cells (PBMCs) from melanoma patients (#02, 05, 07 and 08) were used to assess their reactivity against melanoma associated antigens (MAA)-derived peptides (MAGE-A2, MAGE-A3, NA-17A, NY-ESO-1, MART-1, gp100, TYR) on HLA-A*01+ 1061 EBV-B (Panel A and D) or HLA-A*0201+ T2 cells (Panels A, B and C) and autologous, when available, or allogeneic HLA-matched tumor cell reactivity (Panel C). Interferon γ (IFNγ)-based ELISPOT assay was used for this analysis. Data are expressed as N. of spots/3 × 104cells and are subtracted of the background of IFNγ release from T cells incubated with EBV-B or T2 control cells alone. Results represent averages of triplicates with SD ≤ 10%; statistical analysis of differences between means of IFNγ released by T cells was performed by 2-tailed Student's t-test; significance defined as p < 0.05.
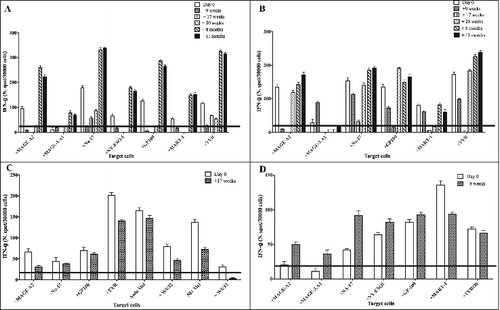
Table 2. Clinical outcome of melanoma patients treated with NGR/Vax/01
Figure 2. Detection of TNF inhibitory soluble factors and VEGF in the serum of melanoma patients. Serum from melanoma patients was collected at different time points: baseline, 2 hours, 1 month and 2 months post-treatments. The presence in the serum of chromogranin A (CgA), soluble tumor necrosis factor (sTNFR) was assessed by ELISA. Statistical analysis of differences between means of soluble factors was performed by 2-tailed Student's t-test with significance defined as p < 0.05.
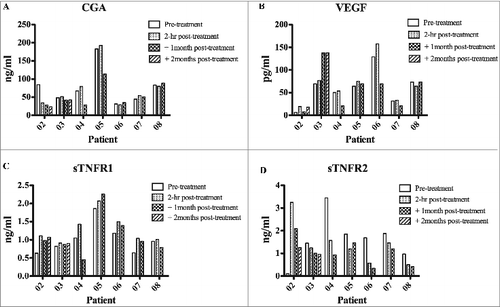
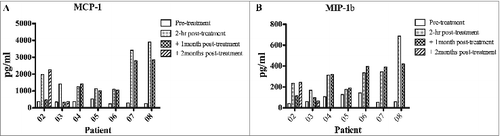
Figure 3. Detection of pro-inflammatory factors (MIP1β and MCP1) in the serum of melanoma patients. Serum from melanoma patients was collected at different time points: baseline, 2 hours, 1 month and 2 months post-treatments. The presence in the serum of macrophage chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 1β (MIP-1β) was assessed by ELISA. Data represent pg/mL of each soluble factor. Statistical analysis of differences between means of soluble factors was performed by 2-tailed Student's t-test with significance defined as p < 0.05.