Figures & data
Table 1. SPAG9 expression, humoral response and clinicopathological characteristics of salivary gland tumor
Figure 1. SPAG9 gene expression in SGT patients. (A) RT-PCR analyses of SPAG9 mRNA expression. SPAG9 transcripts were detected in benign tumor, malignant tumor stage I, stage II, stage III, stage IV and testis. No SPAG9 mRNA was detected in the available four matched ANCT. β-Actin gene expression revealed expression in all the tissues under investigation. (B) RT-PCR analyses of SPAG9 transcript in various histotypes. PA (pleomorphic adenoma), MEC (mucoepidermoid carcinoma), AdCC (adenoid cystic carcinoma), ACC (acinic cell carcinoma), CC (clear cell carcinoma), BCAC (basal cell carcinoma), ANOS (adenocarcinoma not otherwise specified), PLGA (polymorphous low grade adenocarcinoma). No gene expression was detected in the available 8 matched ANCT specimens. β-Actin was used as internal control in these experiments.
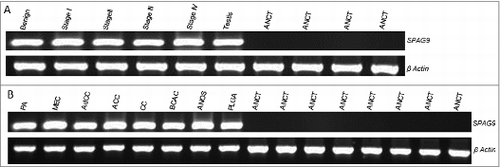
Figure 2. Analysis of SPAG9 gene expression in SGT patients by in situ RNA hybridization. Representative images of H&E staining for benign, malignant stage I, II, III, and IV tumors are shown in left panel. The serial tissue sections probed with anti-sense riboprobes resulted in violet blue color as shown in the middle panel, whereas no hybridization was observed when probed with sense riboprobes as shown in the right panel. Original magnification: x200; objective: x20.
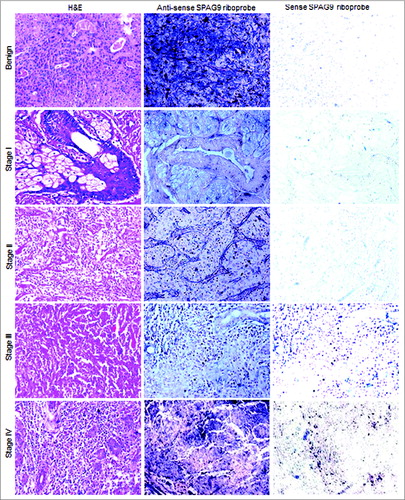
Figure 3. Validation of SPAG9 protein expression in various stages of SGT by immunohistochemistry. First panel shows representative images for H&E staining in benign, malignant stage I, II, III, and IV tumor specimens. Second panel shows the representative images for the cytoplasmic localization of SPAG9 protein probed with anti-SPAG9 antibody as depicted by brown color immunoreactivity. No immunoreactivity was observed in serial tumor sections probed with control IgG, as shown in the third panel. Fourth panel depicts no immunoreactivity against SPAG9 protein in ANCT specimens when probed with anti-SPAG9 antibody. Original magnification: x400; objective: x40.
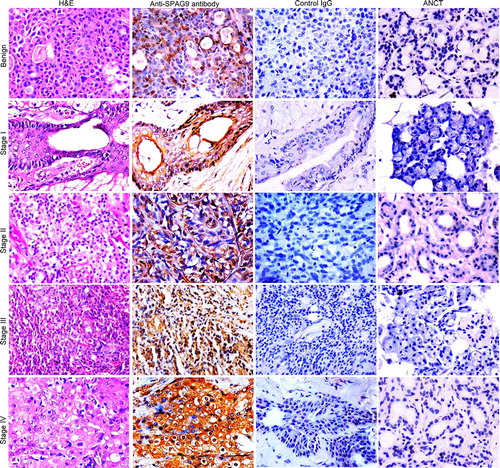
Figure 4. Validation of SPAG9 protein expression in various histotypes of malignant SGT by immunohistochemistry. (A) Top panel showing the cytostructure of representative specimens of benign pleomorphic adenoma and various malignant histotypes such as mucoepidermoid carcinoma, adenoid cystic carcinoma and acinic cell carcinoma of SGT stained with H&E. Middle panel shows cytoplasmic localization of SPAG9 protein expression in the representative specimens of various histotypes of SGT probed with anti-SPAG9 antibody. Bottom panel depicts no immunoreactivity for SPAG9 protein in various histotypes of SGT specimens when probed with control IgG. (B) Top panel showing the cytostructure of representative specimens of various malignant histotypes such as clear cell carcinoma, basal cell carcinoma, adenocarcinoma not otherwise specified and polymorphous low grade adenocarcinoma of SGT stained with H&E. Middle panel shows cytoplasmic localization of SPAG9 protein expression in the representative specimens probed with anti-SPAG9 antibody. Bottom panel depicts no immunoreactivity for SPAG9 protein when probed with control IgG. Original magnification: x400; objective: x40.
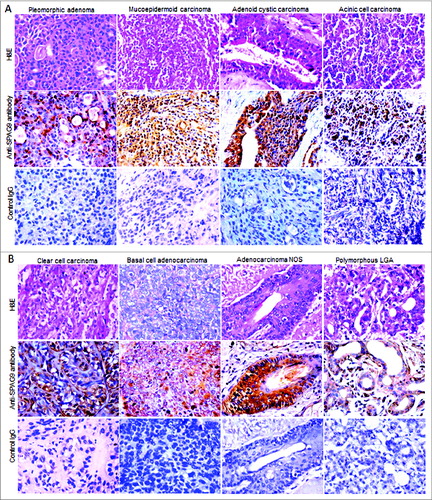
Figure 5. SPAG9 immunoreactivity score. SPAG9 IRS was determined by counting cells (> 500 cells) positive for SPAG9 protein from five random fields in benign, different stages and various histotypes of malignant tumor. The histogram shows benign (PA- pleomorphic adenoma), malignant stage I, stage II, stage III, stage IV, MEC (mucoepidermoid carcinoma), AdCC (adenoid cystic carcinoma), ACC (acinic cell carcinoma), CC (clear cell carcinoma), BCAC (basal cell carcinoma), ANOS (adenocarcinoma not otherwise specified) and PLGA (polymorphous low grade adenocarcinoma). * p < 0.0001 statistically significant, Point indicates mean bars, standard errors.
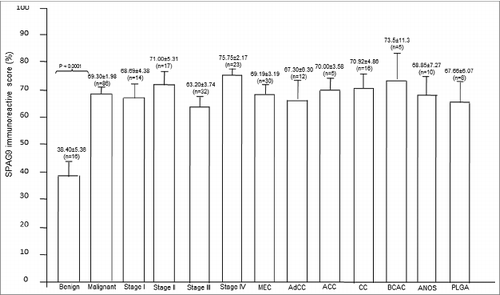
Figure 6. Humoral response against SPAG9 protein in SGT patients. (A) ELISA-based analysis of sera from available 62 SGT patients of benign, different stages (I, II, III and IV) and various histotypes of malignant tumor and from 60 normal healthy donors was carried out to determine the presence of anti-SPAG9 antibodies. The horizontal line X indicates the cutoff value 0.212 (0.137 + 0.074) of 60 normal healthy donors at A492nm. SGT patients were designated as positive for the presence of anti-SPAG9 antibodies above X line and negative below the X line. (B) Western blotting analyses. Lane 1, affinity-purified recombinant SPAG9 stained with Coomassie brilliant blue; lane 2, immuno-blot of recombinant SPAG9 shows a specific band of 170 kDa with anti-SPAG9 antibody (positive control). Serum from patient having benign [PA, (lane 3 and lane 8)], malignant tumor stage I (lane 4), stage II (lane 5), stage III (lane 6), stage IV (lane 7), MEC (lane 9), AdCC (lane 10), ACC (lane 11), CC (lane 12), BCAC (lane 13), ANOS (lane 14), PLGA (lane 15) showed specific immunoreactivity against recombinant SPAG9 protein. Neutralization experiments were done by pre-incubating recombinant SPAG9 protein (15μg/mL) with sera from SGT patients which resulted in complete loss of immunoreactivity (lane 16). Sera from healthy individuals revealed no reactivity (lane 17). M-molecular weight marker. (C) Neutralization studies in serial tissue sections of benign and malignant stages of SGT. Neutralization studies were carried out by pre-incubating anti-SPAG9 antibody generated in rat with recombinant SPAG9 protein (15μg/mL) and used for probing endogenous SPAG9 protein in the serial tissue sections of SGT patients. Left panel shows the representative images of the H&E stained sections of malignant tumor stage I, II, III, IV. Middle panel depicts the SPAG9 protein expression probed with anti-SPAG9 antibodies. Right panel shows complete loss of SPAG9 localization when probed with neutralized sera.
![Figure 6. Humoral response against SPAG9 protein in SGT patients. (A) ELISA-based analysis of sera from available 62 SGT patients of benign, different stages (I, II, III and IV) and various histotypes of malignant tumor and from 60 normal healthy donors was carried out to determine the presence of anti-SPAG9 antibodies. The horizontal line X indicates the cutoff value 0.212 (0.137 + 0.074) of 60 normal healthy donors at A492nm. SGT patients were designated as positive for the presence of anti-SPAG9 antibodies above X line and negative below the X line. (B) Western blotting analyses. Lane 1, affinity-purified recombinant SPAG9 stained with Coomassie brilliant blue; lane 2, immuno-blot of recombinant SPAG9 shows a specific band of 170 kDa with anti-SPAG9 antibody (positive control). Serum from patient having benign [PA, (lane 3 and lane 8)], malignant tumor stage I (lane 4), stage II (lane 5), stage III (lane 6), stage IV (lane 7), MEC (lane 9), AdCC (lane 10), ACC (lane 11), CC (lane 12), BCAC (lane 13), ANOS (lane 14), PLGA (lane 15) showed specific immunoreactivity against recombinant SPAG9 protein. Neutralization experiments were done by pre-incubating recombinant SPAG9 protein (15μg/mL) with sera from SGT patients which resulted in complete loss of immunoreactivity (lane 16). Sera from healthy individuals revealed no reactivity (lane 17). M-molecular weight marker. (C) Neutralization studies in serial tissue sections of benign and malignant stages of SGT. Neutralization studies were carried out by pre-incubating anti-SPAG9 antibody generated in rat with recombinant SPAG9 protein (15μg/mL) and used for probing endogenous SPAG9 protein in the serial tissue sections of SGT patients. Left panel shows the representative images of the H&E stained sections of malignant tumor stage I, II, III, IV. Middle panel depicts the SPAG9 protein expression probed with anti-SPAG9 antibodies. Right panel shows complete loss of SPAG9 localization when probed with neutralized sera.](/cms/asset/3d4e3e8d-9adf-4d6a-94b0-70b11f10352f/koni_a_974382_f0006_oc.jpg)
