Figures & data
Table 1. DTaP vaccines available during the study period (2003–2011) and vaccine brand definitions
Figure 1. Study cohort selection aBrand could not be determined for >95% of trivalent DTaP vaccinations recorded with CPT code 90700. Comparisons between children with complete vs incomplete brand information were made among those with ≥2 DTaP vaccinations. bThe main analysis of DTaP brand mixing was conducted among children with ≥2 DTaP vaccinations and complete brand information. cBetween 2003–2005, trivalent DTaP vaccinations submitted for reimbursement with the code 90700 were changed to a general vaccination code by one of the claims processing systems, therefore some children vaccinated during this period may have incomplete vaccination history. Children with vaccinations processed by the affected system during this time period were excluded to identify a subset with complete DTaP vaccination history for analysis of age-appropriate dose completion and the DTaP brand mixing sensitivity analysis #1.
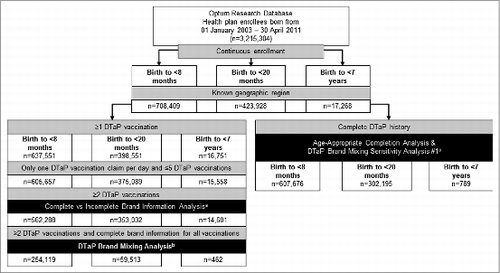
Table 2. Patient and vaccine characteristics of children with complete and incomplete brand information with corresponding P values
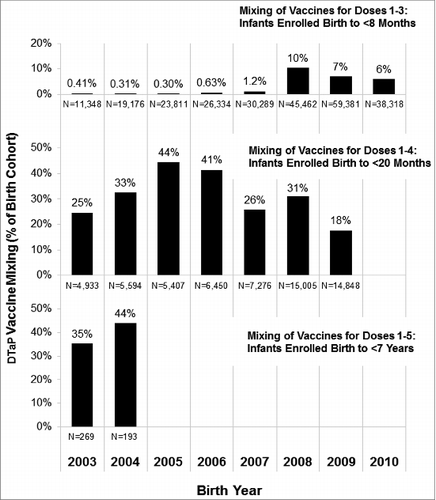
Table 3. Proportion of children with mixing in their DTaP vaccination series
Table 4. DTaP vaccine mixing stratified by patient characteristics within each continuous enrollment cohort among patients with at least 2 administered vaccinations and known brand for all administered doses