Figures & data
Figure 1. Two-dimensional gel electrophoresis proteome map of co-purified APV bulks. Separation and identification of proteins in co-purified APV bulk samples using 2-DE analysis followed by MALDI-TOF/TOF MS. Samples were resolved by IEF (pH 3–11) and 12.5% SDS-PAGE. Protein spots were visualized by colloidal Coomassie staining. The main stained spots were excised individually for identification. Protein spots are labeled as indicated in the legend for . (A) co-purified APV (S2) bulk, from manufacture 1; (B) co-purified APV (S3) bulk from manufacture 2.
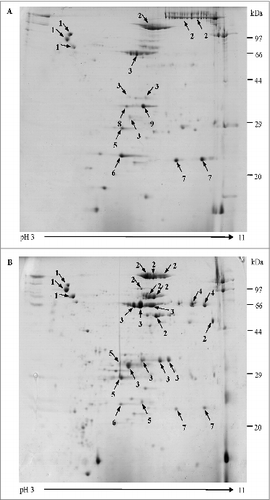
Table 1. Proteins in the co-purified APV bulks identified by mass spectrometry and database research
Figure 2. Levels of antigen-specific IgG in mice immunized with different pertussis vaccines. Sera from control mice and immunized mice were determined by ELISA. Antibody level (EU/mL) is calculated against mouse serum reference NIBSC 97/642. Results represent the geometric mean antibody titres for 5 mice per group. *indicates a statistically significant difference (P < 0.05) compared with WPV (S1) group; # indicates a statistically significant difference (P < 0.05) compared with co-purified APV (S2 and S3) groups.
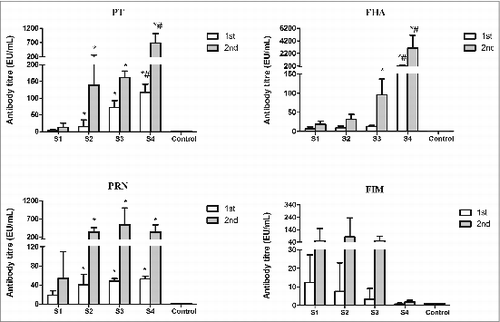
Figure 3. Cytokine responses in mice immunized with different pertussis vaccines. Seven days after the second immunization, spleen cells were prepared from 5 mice from each group. The cytokines were determined by ELISA. Results are the mean responses for 5 mice per group. *indicates a statistically significant difference (P < 0.05) compared with co-purified (S2 and S3) and purified (S4) APV groups. # indicates a statistically significant difference (P < 0.05) compared with purified (S4) APV groups.
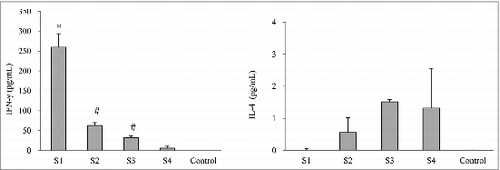
Figure 4. Protective effects of pertussis vaccines in the murine intracelebral challenge model. The results of each test sample were analyzed by the probit method, using the proportion of mice that survived the challenge for estimation of relative potency against Chinese National Standard, and expressed in IU/SHD. Number in bracket is 95% limit.
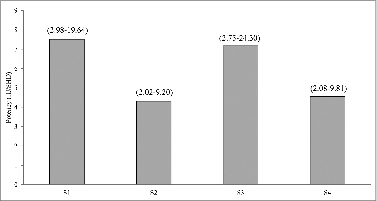
Table 2. Vaccine samples used in this study*
