Figures & data
Table 1. Comparison of different chemiluminescence-based SEAP assay kits
Figure 1. ZiVa can better detect SEAP than GE. Pseudovirion particles of HPV types 16 and 18 were serially diluted, and used to infect 0.03 × 106 293TT cells. Two-fold serial dilutions were performed starting from 1:1000 to 1:1024000. Supernatants were harvested at 48 and 72 hr post-infection. Each PsV dilution was setup in duplicate wells. Mean ± standard deviation (SD) of 3 independent experiments are shown. (A) Infectivity levels (fold-increase) by PsV16 particles after 48 or 72 hr of incubation are shown. (B) Infectivity levels by PsV18 particles after 48 or 72 hr of incubation are shown. In both (A and B), the same supernatant samples were tested using ZiVa or GE SEAP detection kit. The plots show fold-increase in log10 scale as a function of viral concentration (log10). Some of the error bars are too small to be seen in the graphs.
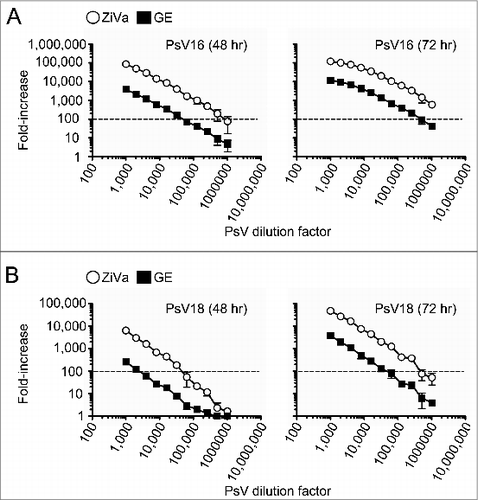
Table 2. Ratios of ZiVa to GE in detecting SEAP
Figure 2. ZiVa can detect SEAP even using limiting numbers of 293TT cells. Different numbers of 293TT cells were plated at the start of the infections by PsV16 or PsV18. The number of cells ranged from 0.002 - 0.3 × 106 cells per well, and 2-fold serial dilutions were performed. Each cell number was tested in triplicates. A fixed dilution factor of 1:100000 for PsV16 (A) and 1:10000 for PsV18 (B) were used to infect 293TT cells. At 48 or 72 hr post-infection, the supernatants were harvested. Cell numbers were plotted in log10 scale. Mean ± SD of 3 independent experiments are shown. Some of the error bars are too small to be seen in the graphs.
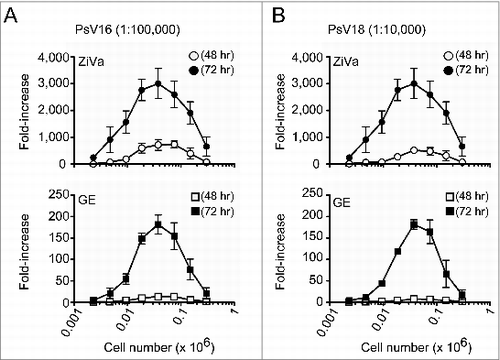
Figure 3. ZiVa can detect SEAP at lower cell numbers using lower viral dilution concentrations. The experiments were conducted as described in , except higher viral dilution factors were used: 1:600000 for PsV16 and 1:60000 for PsV18. Mean ± SD of 3 independent experiments are shown. Some of the error bars are too small to be seen in the graphs.
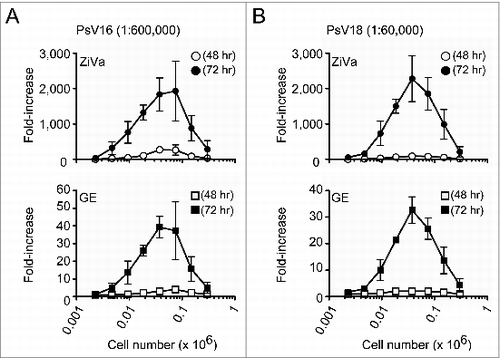
Table 3. Comparison of 50% neutralization titer values measured by GE or ZiVa
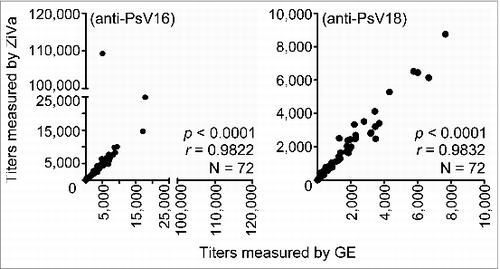
Figure 4. HPV neutralizing antibody titers measured by ZiVa correlate with those of GE. Cell-culture supernatants from 293TT cell-based neutralization assay from the Uganda Immunogenicity Study were used to quantitate 50% neutralization titers in sera of Cervarix®-immunized individuals using ZiVa or GE. The supernatant samples collected after 72 hr of incubation were used to measure the SEAP activity. Spearman rank-correlational analyses were performed between the titers obtained by ZiVa or GE for both anti-HPV16 and -HPV18 antibodies.