Figures & data
Figure 1. Lack of a vaccine-induced lung antibody response in sheep immunized with influenza ISCOMATRIX™ vaccine and challenged intralung with influenza antigen. Study 1: A group of sheep (n = 11) previously vaccinated 3 times via the lung with influenza ISCOMATRIX™ vaccine then challenged with influenza antigen at either 6 or 12 months later,Citation12 were revaccinated via the lungs 18 months after the initial immunizations. Influenza specific antibodies in sera and lung washings collected one week prior to antigen challenge (pre) and one and 4 weeks after antigen challenge were analyzed by ELISA. Age and sex-matched control sheep were sham vaccinated with PBS (n = 12). Boxplots present the median (horizontal bar), interquartile range (box) and range (bars). *ANOVA with Dunnett's post-hoc analysis.
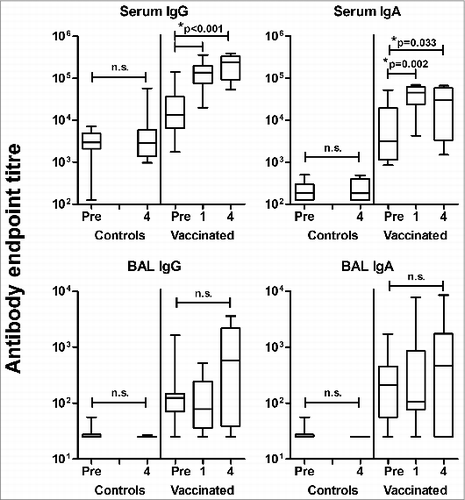
Figure 2. Antigen pre-exposure in the lung is locally immunogenic but reduces the efficacy of subsequent pulmonary influenza ISCOMATRIX™ vaccination. Study 2: (A) Influenza antigen (15 μg) alone was delivered into the lungs of sheep (n = 16). Control sheep (n = 16) were left untreated. Sera and BAL samples collected one week before and 2 weeks (optimal timepoint for measuring priming responses) after antigen delivery were analyzed for influenza-specific antibodies by ELISA. Endpoint titers are shown from post-antigen exposure samples, with titers from pre-exposure samples subtracted. (B) Commencing 3 weeks after influenza antigen exposure in the lung, half of the treated and control groups (n = 8) received 3 influenza ISCOMATRIX™ vaccinations, delivered 3 weeks apart. Sera and BAL samples were collected from all animals one week before and after the final vaccination (optimal timepoint for measuring booster responses) and analyzed for influenza-specific antibodies by ELISA. Endpoint titers shown are from post-vaccination samples with titers from pre-exposure samples subtracted. (C) Neutralizing activity on post-vaccination samples was determined by HAI assay. **P < 0.01; ***P ≤ 0.001 compared with unvaccinated controls (ANOVA).
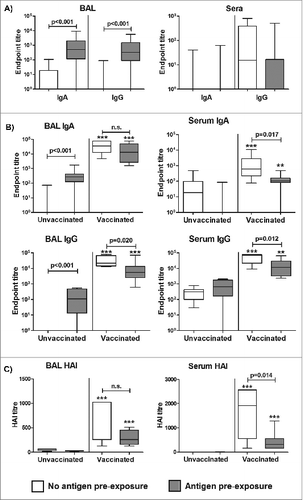
Figure 3. Reduced FOXP3 expression in BAL cells following pulmonary influenza ISCOMATRIX™ vaccinations. Study 2: (A) BAL cells were collected from sheep 1 week after exposure to influenza antigen alone via the lung and a matching group that received no antigen (n = 16). (B) Half of each group received 3 influenza ISCOMATRIX™ vaccinations (Vax) while the other half were left unvaccinated (Con). BAL cells were collected one week after the third vaccination. FOXP3 levels in BAL cells were quantified by qPCR relative to GAPDH (*ANOVA).
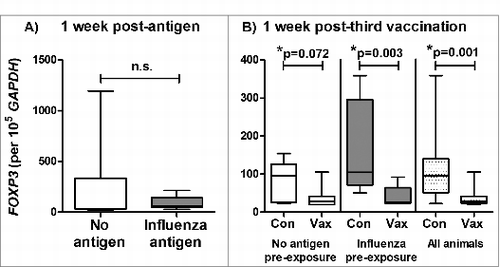
Figure 4. BAL cell composition following lung delivery of influenza antigen or influenza ISCOMATRIX™ vaccine. Study 3: Groups of sheep (n = 8) were left untreated or dosed via the lung with 3 doses of either influenza antigen alone or influenza ISCOMATRIX vaccine, spaced by 3 weeks. One day after the first and third dose, BAL cells were collected from the lungs by endoscopy, stained and counted. (A) Total counts of cells recovered. (B) Relative numbers of macrophages, neutrophils and lymphocytes (%). **P < 0.01; ***P < 0.001 compared to untreated animals.
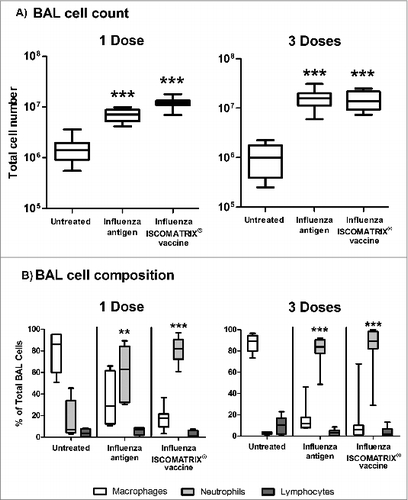
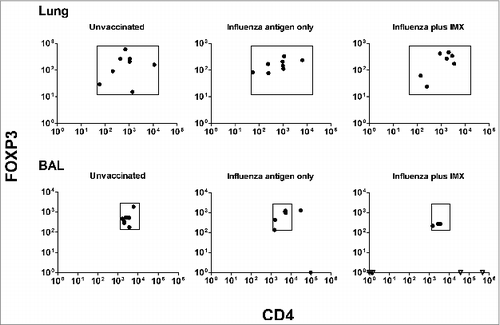
Figure 5. Expression of regulatory T cell markers 1 day after lung delivery of influenza antigen or influenza ISCOMATRIX™ vaccine. Study 3: Groups of sheep (n = 8) were left untreated or dosed via the lung with 3 doses of either influenza antigen alone or influenza ISCOMATRIX™ vaccine, spaced by 3 weeks. One day after the first and third dose, BAL cells and lung tissue samples were analyzed for FOXP3, CD4 and IL-10 by qPCR. *ANOVA.
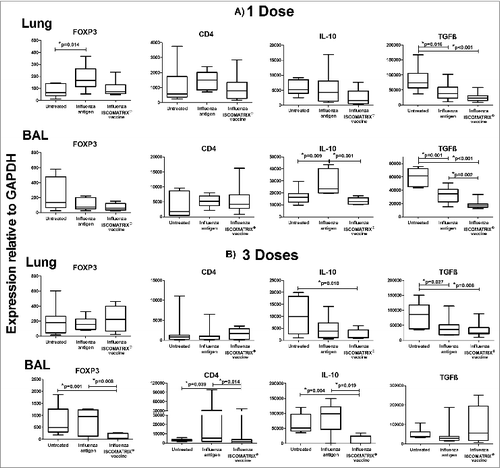
Table 1. Experimental design
Table 2. Primers