Figures & data
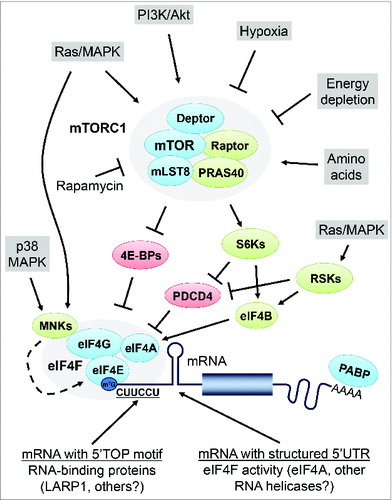
Figure 1. Schematic representation of mTORC1 signaling to the translational machinery. Growth factors and hormones stimulate mTORC1 activity via the Ras/MAPK and PI3K/Akt signaling pathways. mTORC1 is also activated by amino acids, and inactivated by energy depletion and hypoxia. mTORC1 promotes mRNA translation by regulating the 4E-BPs and S6Ks, which in turn modulate downstream effector proteins such as PDCD4 and eIF4B. Phosphorylation of the 4E-BPs by mTORC1 leads to their dissociation from eIF4E, thereby stimulating assembly of the eIF4F complex. The helicase activity associated with the eIF4F complex is thought to be critical for the unwinding of secondary structures within the mRNA 5’UTR, thereby facilitating scanning of the ribosome toward the initiation codon. The S6Ks and RSKs are thought to modulate eIF4A activity by phosphorylating eIF4B and the tumor suppressor PDCD4. The MNKs are recruited via eIF4G and directly phosphorylate eIF4E. mRNAs which contain a 5’ terminal oligopyrimidine (TOP) motif are dependent on mTORC1 activity for their translation. Several RNA-binding proteins were suggested to bind to the TOP motif and regulate TOP mRNA translation, including the La-related protein 1 (LARP1).