Figures & data
Figure 1. Np95 is expressed abundantly in the brain at midgestation, when a large number of NS/PCs exist. (A) Confirmation of antibody reactivity to mouse Np95. HEK293T cells were transfected with FLAG-tagged Np95 expression plasmid and immunoblotted with anti-FLAG, -Np95 and -UHRF1 antibodies. (B) Western blot analysis of mouse whole brain at various stages of development, from E11 to postnatal day 7 (P7) and in 8-week-old adults, using anti-DNMT1, -Np95 and -actin antibodies. Np95 and DNMT1 bands were strongest in E11 brains. (C) Representative immunofluorescence images for Np95, Ki67 and Sox2 in E14 mouse forebrain sections. Scale bar: 250 μm. (D) Confocal immunofluorescence images for Np95, Ki67 and Sox2 in E14 mouse forebrain sections. Scale bars: 10 μm. (E–G) Representative immunofluorescence images of E14 mouse forebrain sections. Immunostaining of Np95 (red), Nestin (cyan) and Ki67 (E), DCX (F) or MAP2ab (G) (green). The insets in Merge images show Hoechst staining. Np95 expression was observed only in Ki67+ or Nestin+ proliferating NS/PCs. Scale bars: 100 μm. VZ: ventricular zone; SVZ: subventricular zone; IZ: intermediate zone; CP: cortical plate.
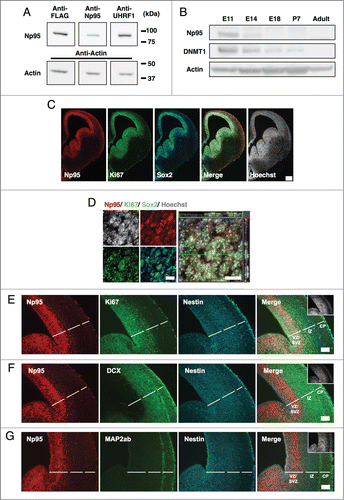
Figure 2. Np95 is not expressed in differentiated neural cells. (A–D) Representative immunofluorescence images of cells cultured under various conditions. Neuroepithelial cells from E14 mice were cultured with bFGF (10 ng/ml) for 4 days, and then cultured as described below, for the indicated number of days, either to maintain the undifferentiated condition or to induce specific differentiation. (A) Undifferentiated cells: bFGF (10 ng/ml) for 2 d. (B) Neurons: 0.5% FBS for 4 d. (C) Astrocytes: bFGF (10 ng/ml), leukemia inhibitory factor (LIF) (40 ng/ml) and bone morphogenetic protein 2 (BMP2) (40 ng/ml) for 4 d. (D) Oligodendrocytes: triiodothyronine (T3) (30 ng/ml) and thyroxin (T4) (40 ng/ml) for 7 d. After each differentiation induction period, the cells in (A-D) were stained with antibodies against Np95 (A–D) (red) and either Ki67, Nestin (A), Tuj1 (B), GFAP (C) or APC (D) (green). Np95 expression was not observed in differentiated neural cells. The insets in Merge images show Hoechst staining. White arrowheads indicate representative Np95-expressing cells. Scale bars: 20 μm.
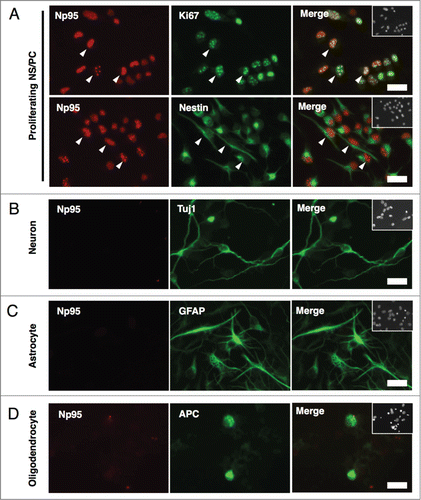
Figure 3. Np95 is preferentially expressed in actively dividing type 2a cells in the adult hippocampus. (A) Schematic diagram of marker proteins that are expressed in the hippocampal granule cell layer during the 5 discernible stages of adult hippocampal neurogenesis. (B) Representative confocal immunofluorescence images for Np95 and Ki67 in the adult mouse hippocampal DG. Scale bars: 20 μm. (C) Ratio of Np95+/Ki67+ cells to total Np95+ cells or to total Ki67+ cells in hippocampal DG. While 97% of Np95-expressing cells exhibited a Ki67 signal, 30% of Ki67-expressing cells did not exhibit an Np95 signal. Error bars represent the mean ± SEM (n = 3). (D) Representative immunofluorescence images of the adult hippocampal DG. Immunostaining for Np95 (red, left panel), Ki67 (green, left middle panel) and Nestin, GFAP or DCX (cyan, right middle panel). Scale bars: 50 μm. The insets in Merge images show Hoechst staining. White arrowheads indicate representative Np95-, Ki67- and specific cell marker (Nestin-, GFAP-, DCX-) expressing cells. (E) Identification of Np95-expressing cell types in the DG of the adult hippocampus. Ki67+ cells, Ki67+/Np95+ cells and Ki67+/Np95- cells were counted using the merged images of (D). The bar graphs indicate the percentages of Nestin+/− (left), GFAP+/− (middle) or DCX+/− (right) cells among Ki67+ cells, Ki67+/Np95+ cells and Ki67+/Np95- cells in the hippocampal DG. The bar colors correspond to the stage colors used in the illustration in (A). Values are given as mean ± SEM. One-way ANOVA (Prism, GraphPad) followed by Tukey test: n = 3; *p < 0.05, **p < 0.01, ***p < 0.001.
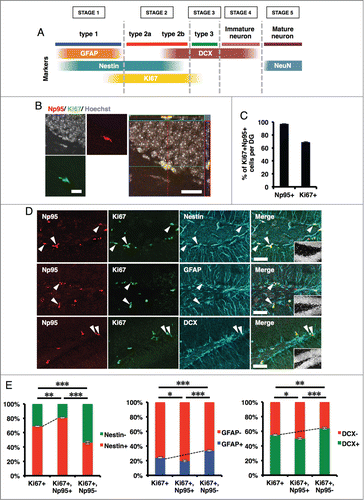
Figure 4. KA injection increases the number of Np95-expressing cells. (A) Experimental scheme for inducing seizure. 8-week-old mice were injected intraperitoneally with KA and sacrificed 4 d later. (B) Representative immunofluorescence images of Np95 and Ki67 staining in control and KA-injected mice. Merge images show higher-magnification views of the white boxes in the middle images. Scale bars: 100 μm in Np95 and Ki67 images, 50 μm in Merge images. (C) Quantification of the number of Ki67+ cells in the hippocampal DG of control and KA-injected mice. KA injection increased the number of Ki67+ cells in the DG. Values are given as mean ± SEM. Student's t-test: n = 3; **p < 0.01. (D) Quantification of the number of Ki67+/Np95+ cells in the DG of control and KA-injected mice. KA injection increased the number of Ki67+/Np95+ cells in the DG. Values are given as mean ± SEM. Student's t-test: n = 3; **p < 0.01. (E) Ratio of Np95+/Ki67+ cells to total Ki67+ cells in the DG. Values are given as mean ± SEM. Student's t-test: n = 3; n.s., not significant.
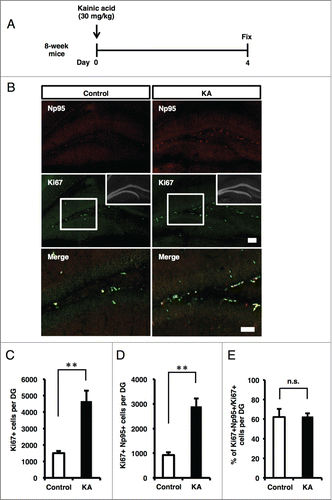
Figure 5. Schematic representation of the expression pattern of Np95 in embryonic brain and adult hippocampal DG. (A) NS/PCs acquire the pluripotency to differentiate into neurons, astrocytes and oligodendrocytes during embryonic development. Np95 is specifically expressed in NS/PCs and not expressed in their completely differentiated neural cell progeny. (B) Schematic diagram illustrating the current view of lineage relationships and marker expression during adult hippocampal neurogenesis. Adult hippocampal neurogenesis occurs in 5 stages: [1] activation of quiescent radial glia-like cells (type 1 (change of morphology from radial to horizontal)) in the SGZ; [2] proliferation of precursor and intermediate progenitors (type 2a, 2b, transit-amplifying cells); [3] generation of neuroblasts (type 3); [4] differentiation into immature neurons; [5] maturation of immature neurons. Np95 is preferentially expressed in type 2a transit-amplifying NS/PCs. Circular arrows indicate the frequency of cell division. Solid arrows show frequently dividing cells compared with dashed arrows. ML: molecular layer; GCL: granule cell layer; SGZ: subgranular zone.
![Figure 5. Schematic representation of the expression pattern of Np95 in embryonic brain and adult hippocampal DG. (A) NS/PCs acquire the pluripotency to differentiate into neurons, astrocytes and oligodendrocytes during embryonic development. Np95 is specifically expressed in NS/PCs and not expressed in their completely differentiated neural cell progeny. (B) Schematic diagram illustrating the current view of lineage relationships and marker expression during adult hippocampal neurogenesis. Adult hippocampal neurogenesis occurs in 5 stages: [1] activation of quiescent radial glia-like cells (type 1 (change of morphology from radial to horizontal)) in the SGZ; [2] proliferation of precursor and intermediate progenitors (type 2a, 2b, transit-amplifying cells); [3] generation of neuroblasts (type 3); [4] differentiation into immature neurons; [5] maturation of immature neurons. Np95 is preferentially expressed in type 2a transit-amplifying NS/PCs. Circular arrows indicate the frequency of cell division. Solid arrows show frequently dividing cells compared with dashed arrows. ML: molecular layer; GCL: granule cell layer; SGZ: subgranular zone.](/cms/asset/9b9470db-b0f3-4efd-9edc-453c169b150d/kngs_a_976026_f0005_c.gif)
