Figures & data
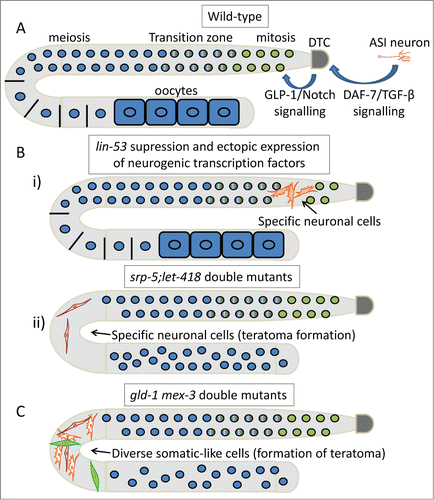
Figure 1. Apoptosis prevents tumorigenesis. Schematic representation of DNA damage-induced apoptosis that limits tumor growth in mammals and C. elegans. In mammals, activation of the proapoptotic proteins Bax and Bak under stress leads to release of cytochrome c. In turn, cytochrome c interacts with Apaf1, which initiates the activation of a caspase cascade. Defects in apoptosis promote tumorigenesis. In C. elegans, binding of EGL-1 to CED-9/CED-4 complex on the surface of mitochondria releases mitochondrial components allowing CED-4 to activate CED-3. CED-9, C. elegans homolog of antiapoptotic BCL-2; CED-4, C. elegans homolog of apoptotic protease activating factor 1 (APAF-1).
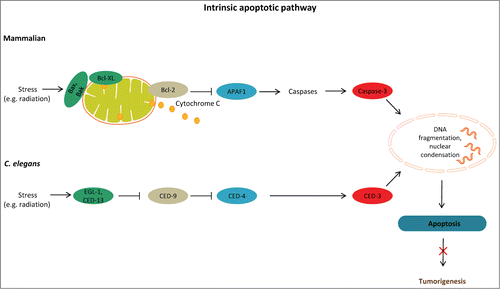
Figure 2. Linking cell death mechanisms to cancer. Deregulation of cell death mechanisms contributes to pathologic conditions, including cancer. Basal autophagy supports metabolism through recycling of cytoplasmic material and serves a quality control function through protein and organelle turnover. Impaired autophagy leads to accumulation of damaged organelles and protein aggregates, thereby promoting cellular damage and increased vulnerability to disease. Stressful conditions, such as low nutrient availability, energy depletion, hypoxia, and oxidative stress, induce autophagy. Excessive autophagy (for example due to activation of AMPK) can also be detrimental. Longevity-influencing genes have a role in tumor suppression; for example, DAF-16/FOXO target genes can induce tumor cell apoptosis and prevent tumor growth. Arrows indicate stimulatory inputs. Bars indicate inhibitory interactions. For clarity, some of the signaling connections are not shown. FOXO/DAF-16, a forkhead box O(FOXO) transcription factor; HIF-1, hypoxia inducible factor-1; LKB1, serine/threonine protein kinase; TOR, target of rapamycin; TSC1/2, tuberosclerosis complexes 1 and 2; ROS, reactive oxygen species.
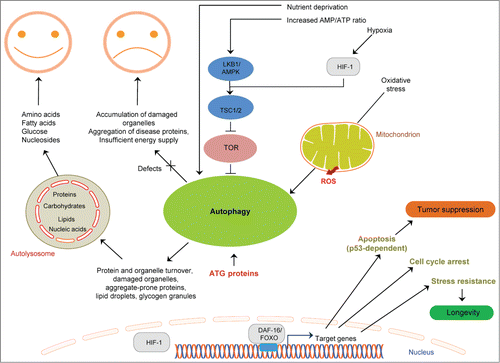
Figure 3. Mechanisms for stem cell maintenance, differentiation and reprogramming derived from studies in C. elegans. (A) A wild-type gonad of a hermaphrodite worm. GLP-1/Notch signaling from the DTC cell is required for normal germ cell renewal. Neuroendocrine effects mediated by ASI-expressed DAF-7/TGF-β are also implicated. (B) Examples of how epigenetic factors may influence reprogramming. i) Reduction of a histone chaperone (LIN-53) and concomitant ectopic expression of a specific neurogenic transcription factor leads to differentiation of germ cells into specific neuronal ones (depending on the neurogenic factor expressed). ii) Germ cells are reprogrammed into specific neurons in SRP-5 (a histone demethylase) and LET-418 (a chromatin remodeler) double mutants. (C) Example of germ cell reprogramming mediated by RNA. GLD-1 and MEX-3 are RNA binding proteins that regulate germ cell differentiation.