Figures & data
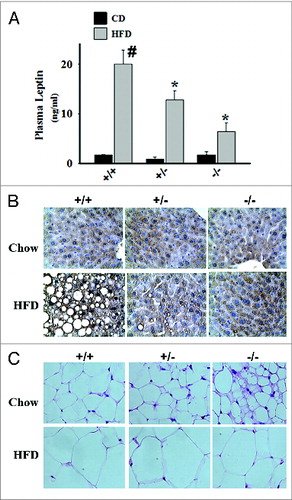
Figure 1. Schematic diagram showing adipose tissue dynamics in lean and obese states. Mesenchymal stem cells generate committed preadipocytes that differentiate into unilocular white and multilocular beige (with increased mitochondrial content) adipocytes in response to adipogenic signals to maintain metabolic homeostasis. Chronic high fat diet (HFD)-induced obesity impairs adipogenesis, promotes enlargement of individual adipocytes, decreases beige adipocyte abundance, and increases adipose tissue inflammatory cells, leading to insulin resistance and other consequences of metabolic disease. Decline in histone deacetylase 9 (HDAC9) level precedes activation of the differentiation program, while elevated HDAC9 levels in HFD fed condition impairs activation of this program, promoting adipocyte hypertrophy.
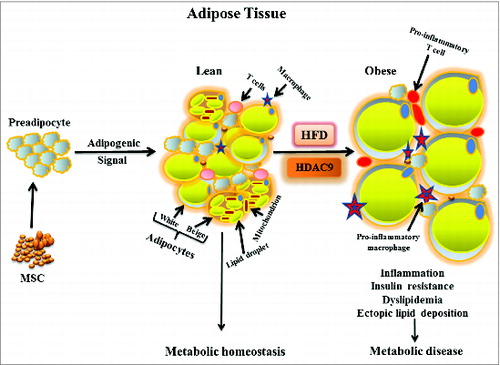
Figure 2. Effects of HDAC9 gene deletion on plasma leptin levels, hepatic lipid accumulation, and multilocular beige adipocyte abundance during chronic high fat diet. (A) Compared with chow diet, feeding 8-wk old male C57BL/6J mice with a high fat diet (60% calories from fat) for 12 wk dramatically increases plasma leptin levels. Deletion of one or both alleles of the HDAC9 gene causes a gene-dose dependent reduction in plasma leptin levels in these mice. (B) Chronic high fat diet causes significant hepatic lipid accumulation in wild type mice, which is prevented by the deletion of one or both alleles of HDAC9 gene. (C) HDAC9 gene deletion (–/–) noticeably increased the abundance of multilocular beige adipocytes in subcutaneous adipose tissue, especially in chow fed mice. Data are mean ± SEM of 4 experiments; #P < 0.05 compared with chow fed wild-type (+/+) mice, *P < 0.05 compared with high fat fed wild-type (+/+) mice.