Figures & data
Figure 1. The cyclooxygenase (COX) pathway. COX exists in two different isoforms (COX-1 and COX-2) and oxygenates arachidonic acid to form prostaglandin (PG) G2 that is further reduced to PGH2. PGH2 is a highly unstable endoperoxide that is rapidly converted by specific synthases to PGs of the E, F and D series and also to PGI2 (prostacyclin) and thromboxane (TX) A2. Both PGI2 and TXA2 have very short half-lifes and are rapidly hydrolyzed to the inactive compounds 6-keto-PGF1α and TXB2, respectively. PGD2 undergoes nonenzymatic dehydration, losing water to form the cyclopentenone 15-deoxy-Δ12-14-PGJ2 (15d-PGJ2). The biological effects of PGs are mediated by ten different types and subtypes of receptors, which belong to the G protein-coupled rhodopsin-type receptor superfamily of seven transmembrane domains. Four of the receptor subtypes bind PGE2 (EP1, EP2, EP3 and EP4), two bind PGD2 (DP1 and DP2), two bind TXA2 (TPα and TPβ) and the rest are single receptors for PGF2α and PGI2 (FP and IP, respectively). 15d-PGJ2 is a natural ligand of PPARγ.
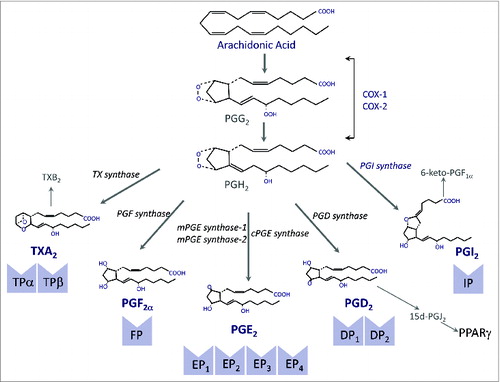
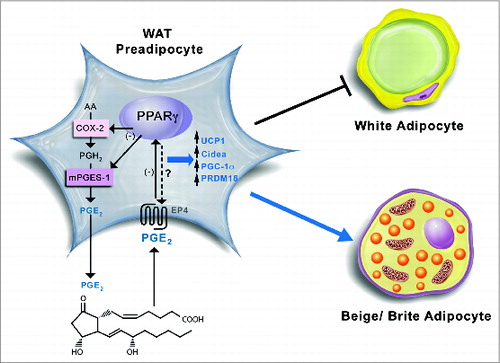
Figure 2. Schematic diagram of the proposed coordinated functional regulation of microsomal prostaglandin E (PGE) synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in beige/brite adipogenesis in white adipose tissue (WAT). In WAT preadipocytes, mPGES-1 cooperates with cyclooxygenase-2 (COX-2) in the biosynthesis and release of prostaglandin (PG) E2 from arachidonic acid. By binding to its receptors (presumably PGE2 receptor EP4 subtype), PGE2 is able to downregulate PPARγ expression, which in turn suppresses COX-2 and mPGES-1 expression in an autocrine fashion. Furthermore, the interaction between PGE2 and PPARγ has the ability to induce brown adipogenic genes such as uncoupling protein 1 (UCP1), cell death-inducing DFFA-like effector a (CIDEA), PPARγ co-activator-1α (PGC-1α), and PR domain containing 16 (PRDM16), which divert pre-adipocyte differentiation into beige/brite adipocytes instead of white adipocytes.