Figures & data
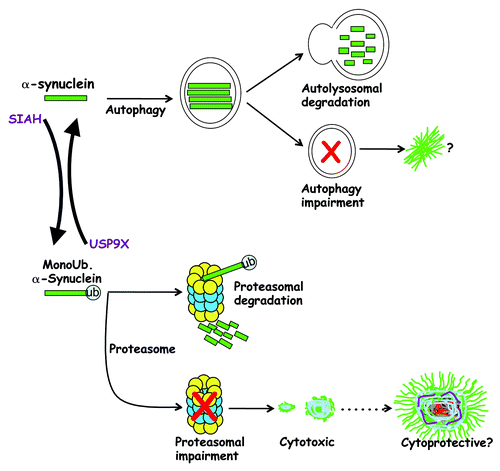
Figure 1. Monoubiquitination determines α-synuclein fate. USP9X deubiquitinates α-synuclein, and nonubiquitinated α-synuclein is mostly degraded by the autophagy pathway. Autophagy impairment may favor α-synuclein inclusion formation. SIAH monoubiquitinates α-synuclein, which leads to its degradation by the proteasome. Cytosolic USP9X is decreased in α-synucleinopathies and this will contribute to the accumulation of monoubiquitinated α-synuclein. Since monoubiquitinated α-synuclein is more prone to aggregation, it may contribute to Lewy body formation caused by proteasomal/autophagy dysfunction. Although monoubiquitination of α-synuclein promotes the formation of toxic inclusions, it is still not clear if Lewy bodies are cytotoxic or cytoprotective.