Figures & data
Figure 1. Serum starvation (SS) in EC induces both apoptotic and autophagic features. (A and B) Electron micrographs of normal and apoptotic EC. The nucleus (Nu) of EC exposed to complete growth medium (Normal) displays an elongated aspect whereas that of serum-starved EC (SS) for 4 h appears rounded and condensed, characteristic of apoptosis. Scale bar: 1 µm. (C) Percentages of cells with increased chromatin condensation (apoptosis) and cell membrane permeabilization (necrosis), as evaluated by HO and PI staining, in EC exposed to normal medium (N) or SS for 1–4 h. *p ≤ 0.0007 vs. N, n = 6. (D) Immunoblot for uncleaved and cleaved forms of PARP in EC treated as in (C), representative of 4 experiments. (E) Upper panel: Time-course of LC3 turnover, by immunoblot, in EC treated as in (A–D). Lower panel: Densitometry analysis of LC3-II/LC3-I ratios in EC exposed to N or SS for 1–4 h. *p ≤ 0.01 vs. N. Representative of four experiments. (F) LC3-I and -II immunoblot in EC exposed to N, SS and SS + bafilomycin A1 5 nM for 4 h (the immunoblot corresponds to two parts of the same gel). Representative of four experiments. (G–K) Morphological characterization of AV in EC serum starved for 4 h. (G) AV with intact cytoplasmic portions or organelles delimited by multiple membranes with internal electron-lucent material and cytoplasm. (H and I) AV with various stages of degraded cytoplasmic material, characterized by increased electron density within vacuoles surrounded by single or double delimiting membranes. (J) AV displaying a more advanced degradation stage with multilamelar lysosomal bodies (white star). (K) Amphisomes are characterized by the presence of both autophagosomal electron-dense material with a delimiting membrane and MVB nanovesicular content. Scale bar: 0.5 µm. (L) Total cytoplasmic area (µm2) occupied by AV per cell profile in EC exposed for 4 h to N and SS, respectively. Area of AV per cell profile (n = 20) was assessed in relation to the cell cytoplasm (nuclei were not included in the evaluation); *p ≤ 0.001 vs. N.
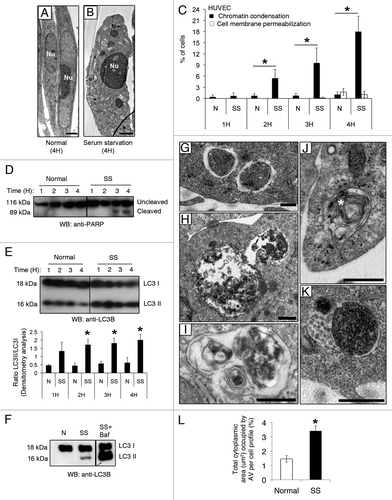
Figure 2. Caspase inhibition does not prevent development of autophagy in serum-starved EC. (A) Percentages of cells with increased chromatin condensation (apoptosis) and cell membrane permeabilization (necrosis), as evaluated by HO and PI staining, in EC exposed to SS in presence of the pan-caspase inhibitor ZVAD-FMK (SS+ZVAD) 50 µM or SS + vehicle DMSO (SS) for 1–4 h. *p ≤ 0.002 vs. Z, n = 6. (B) Immunoblot for uncleaved and cleaved PARP in EC treated as described above. Representative of 4 experiments. (C and D) Electron micrographs of EC serum starved for 4 h with vehicle DMSO [SS, (C)] or with ZVAD-FMK 50 µM [SS+ZVAD, (D)]. Scale bar: 1 µm. (E) Mean cytoplasmic area of the cell profiles. (F) Percentage of total cytoplasmic area (µm2) occupied by AV per cell profile in EC treated as in (A–D). *p = 0.01 vs. SS; n = 30 cell profiles. (G) Mean number of AV profiles per cell profile in EC serum starved for 4 h in presence of the pan-caspase inhibitor ZVAD-FMK 50 µM (SS+ZVAD) or vehicle DMSO (SS). *p ≤ 0.0005 and **p ≤ 0.005 vs. SS; n = 30 cell profiles. (H) Time course of LC3 turnover by immunoblot in EC treated as in A (the immunoblot corresponds to two parts of the same gel). Representative of three experiments. (I–K) Native LC3 immunostaining evaluated by confocal microscopy in EC exposed either to (I) normal medium (N) or serum-starved and treated with (J) vehicle DMSO (SS) or (K) ZVAD-FMK 50 µM (SS+ZVAD). (L) Mean number of LC3 puncta per cell profile evaluated by confocal microscopy immunostaining in EC treated as above. LC3 puncta were counted in approximatly 50 cells/representative section of the sample in three different trials; p ≤ 0.0001. (M) Upper panel: LC3-I and -II immunoblot in EC exposed to vehicule (SS), SS+ZVAD (50 µM), SS + bafilomycin A1 (5 nM) or SS + ZVAD + bafilomycin A1 for 4 h. Lower panel: Densitometry analysis of the LC3-II/LC3-I ratio. *p ≤ 0.02 vs. SS, **p ≤ 0.0002 vs SS+ZVAD. Representative of four experiments.
![Figure 2. Caspase inhibition does not prevent development of autophagy in serum-starved EC. (A) Percentages of cells with increased chromatin condensation (apoptosis) and cell membrane permeabilization (necrosis), as evaluated by HO and PI staining, in EC exposed to SS in presence of the pan-caspase inhibitor ZVAD-FMK (SS+ZVAD) 50 µM or SS + vehicle DMSO (SS) for 1–4 h. *p ≤ 0.002 vs. Z, n = 6. (B) Immunoblot for uncleaved and cleaved PARP in EC treated as described above. Representative of 4 experiments. (C and D) Electron micrographs of EC serum starved for 4 h with vehicle DMSO [SS, (C)] or with ZVAD-FMK 50 µM [SS+ZVAD, (D)]. Scale bar: 1 µm. (E) Mean cytoplasmic area of the cell profiles. (F) Percentage of total cytoplasmic area (µm2) occupied by AV per cell profile in EC treated as in (A–D). *p = 0.01 vs. SS; n = 30 cell profiles. (G) Mean number of AV profiles per cell profile in EC serum starved for 4 h in presence of the pan-caspase inhibitor ZVAD-FMK 50 µM (SS+ZVAD) or vehicle DMSO (SS). *p ≤ 0.0005 and **p ≤ 0.005 vs. SS; n = 30 cell profiles. (H) Time course of LC3 turnover by immunoblot in EC treated as in A (the immunoblot corresponds to two parts of the same gel). Representative of three experiments. (I–K) Native LC3 immunostaining evaluated by confocal microscopy in EC exposed either to (I) normal medium (N) or serum-starved and treated with (J) vehicle DMSO (SS) or (K) ZVAD-FMK 50 µM (SS+ZVAD). (L) Mean number of LC3 puncta per cell profile evaluated by confocal microscopy immunostaining in EC treated as above. LC3 puncta were counted in approximatly 50 cells/representative section of the sample in three different trials; p ≤ 0.0001. (M) Upper panel: LC3-I and -II immunoblot in EC exposed to vehicule (SS), SS+ZVAD (50 µM), SS + bafilomycin A1 (5 nM) or SS + ZVAD + bafilomycin A1 for 4 h. Lower panel: Densitometry analysis of the LC3-II/LC3-I ratio. *p ≤ 0.02 vs. SS, **p ≤ 0.0002 vs SS+ZVAD. Representative of four experiments.](/cms/asset/3b53dd45-0ce5-4176-a665-a535cea6f35b/kaup_a_10919768_f0002.gif)
Figure 3. Caspase activation regulates the formation of autophagic network. (A–E) Electron micrographs of serum-starved EC exposed to ZVAD-FMK 50 µM (SS+ZVAD) or vehicle (SS) for 4 h. (A and B) Autophagic network (islet in A) with different maturation stages observed in serum-staved EC. Multilamellar electron-dense lysosomal bodies are also represented (white stars). White arrowheads indicate contact sites between AV. Scale bars: (A) 0.5 µm; (B) 2 µm. (C–E) Electron micrographs of EC serum starved with ZVAD-FMK 50 µM (SS+ZVAD) showing individual AV (islet in D and E) as opposed to large autophagic network seen in (B). Scale bars: (C) 0.5 µm; (D and E) 2 µm. (F) Mean number of contact sites per AV profile quantified for each condition; n = 30 cell profiles per condition; p = 0.005.
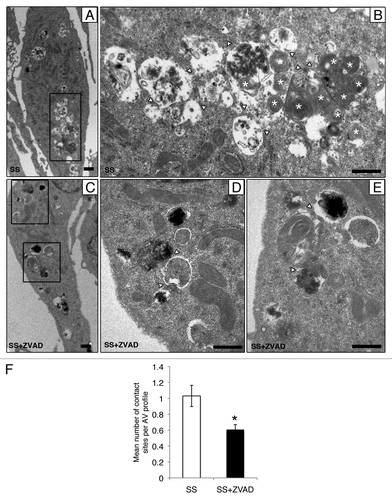
Table 1. List of autophagy-related proteins identified by LC-MS-MS in media conditioned by serum-starved, caspase-activated EC (SSC) and serum-starved, caspase-inhibited EC (SSC-ZVAD)
Figure 4. Caspase-dependent release of AV components during serum starvation (A) Immunoblot analysis for LAMP2, LC3-I, LC3-II and ATG16L1 in 25 ml of media conditioned by EC serum staved with vehicle DMSO (SSC) or with ZVAD-FMK 50 µM (SSC ZVAD); n = 3 for ATG16L1, LC3I and LC3-II; n = 2 for LAMP2. (B–E) Electron micrographs of EC serum starved with vehicle for 4 h showing AV near and/or interacting with the cell membrane. Scale bar: (B and C) 0.5 µm; (D) 1 µm. (E) Islet of the autophagic network in (D) located near the cell membrane; contact sites (white arrowheads). Scale bar: 0.5 μm. (F) AV in EC serum starved incubated with ZVAD-FMK 50 µM (SS + ZVAD) are located farther away from the cell membrane as opposed to AV seen in EC serum starved with vehicle (SS) (D and E). Scale bar: 1 µm. (G) Islet of the AV in (F). Scale bar: 0.5 µm. (H) Distance (µm) of AV from the cell membrane in serum-starved EC treated as in (A–G). Each AV distance was measured perpendicularly to the cell membrane in electron micrographs using ImageJ software; n = 30 cell profiles for each condition *p < 0.001 vs. SS.
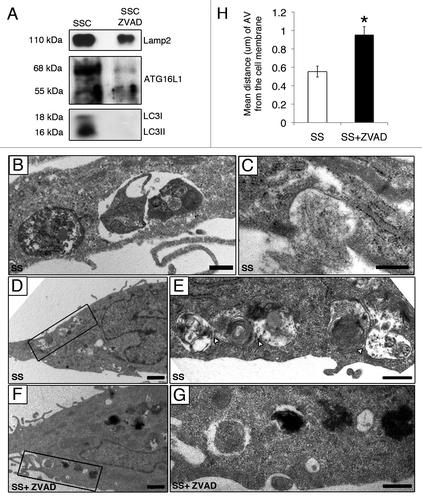
Figure 5. Caspase-3-dependent release of AV components in serum-starved murine EC. (A) Percentages of cells with chromatin condensation and cell membrane permeabilization (as evaluated by HO and PI staining) in aortic EC isolated from controls (WT) and CASP3-deficient (C3−/−) mice exposed to normal medium 4 h or SS for 2–4 h. *p ≤ 0.02 vs. WT, n = 6. (B) Upper panel: Immunoblot for LC3-I/-II in EC treated as described above. Lower panel: densitometry analysis of LC3-II/LC3-I ratios for WT and C3−/− murine EC serum starved for 4 h. Representative of 16 independent WT mice and 6 C3−/− mice. (C–E) Electron micrographs of control murine EC (WT) exposed to SS for 4 h showing AV near and/or interacting with the cell membrane [islet in (E)]. Scale bars: (C and D) 2 µm; (E): 0.5 µm. (F–H) Electron micrographs of murine C3−/− EC exposed to SS for 4 h showing enhanced autophagic vacuolization located away from the cell membrane (islet in H) as compared with WT (E). Scale bars: (F and G) 2 µm; (H) 0.5 µm. (I) Upper panel: Immunoblot analysis for LC3-I and LC3-II in 10 ml of serum-free media conditioned by controls (SSC WT) or CASP3-deficient (SSC C3−/−) murine EC. Lower panel: Densitometry analysis of LC3-I and LC3-II; n = 3.
![Figure 5. Caspase-3-dependent release of AV components in serum-starved murine EC. (A) Percentages of cells with chromatin condensation and cell membrane permeabilization (as evaluated by HO and PI staining) in aortic EC isolated from controls (WT) and CASP3-deficient (C3−/−) mice exposed to normal medium 4 h or SS for 2–4 h. *p ≤ 0.02 vs. WT, n = 6. (B) Upper panel: Immunoblot for LC3-I/-II in EC treated as described above. Lower panel: densitometry analysis of LC3-II/LC3-I ratios for WT and C3−/− murine EC serum starved for 4 h. Representative of 16 independent WT mice and 6 C3−/− mice. (C–E) Electron micrographs of control murine EC (WT) exposed to SS for 4 h showing AV near and/or interacting with the cell membrane [islet in (E)]. Scale bars: (C and D) 2 µm; (E): 0.5 µm. (F–H) Electron micrographs of murine C3−/− EC exposed to SS for 4 h showing enhanced autophagic vacuolization located away from the cell membrane (islet in H) as compared with WT (E). Scale bars: (F and G) 2 µm; (H) 0.5 µm. (I) Upper panel: Immunoblot analysis for LC3-I and LC3-II in 10 ml of serum-free media conditioned by controls (SSC WT) or CASP3-deficient (SSC C3−/−) murine EC. Lower panel: Densitometry analysis of LC3-I and LC3-II; n = 3.](/cms/asset/737c2543-6a59-4d7a-ab61-839d6d82e90e/kaup_a_10919768_f0005.gif)