Figures & data
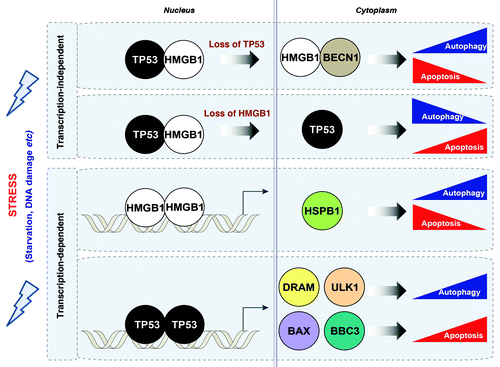
Figure 1. Relationship between HMGB1 and TP53 in the crossregulation of apoptosis and autophagy. HMGB1 and TP53 play transcription-dependent and -independent roles in the regulation of autophagy and apoptosis. Stress signals including serum starvation and DNA damage promote interactions between TP53 and HMGB1 in the nucleus and cytoplasm. Loss of TP53 increases cytosolic HMGB1 leading to increased binding to BECN1, thereby promoting autophagy, and decreasing apoptosis. In contrast, loss of HMGB1 increases cytosolic TP53 and apoptosis, and decreases autophagy. In the nucleus, HMGB1 regulates expression of heat shock protein β-1 (HSPB1/HSP27). HSPB1 inhibits apoptosis through an ability to interact with key components of the apoptotic signaling pathway, as we have recently shown. HSPB1 and its phosphorylation promotes autophagy through regulation of the actin cytoskeletal response, which is required to promote the processes of autophagy and mitophagy. DRAM and ULK1 have recently been identified as TP53 transcriptionally-regulated genes through which TP53 promotes autophagy in contrast to its cytosolic role. In addition, BAX and the TP53 upregulated modulator of apoptosis (BBC3/PUMA) are TP53-targeted genes that induce the mitochondrial apoptotic pathway.