Figures & data
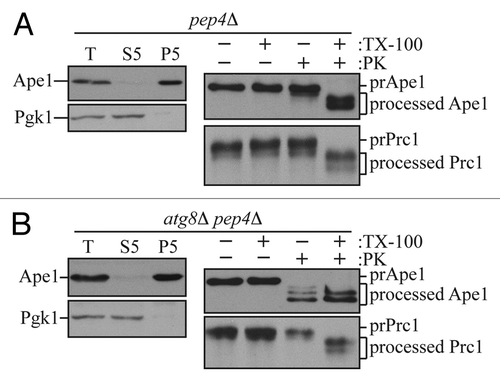
Figure 1. The prApe1 proteinase protection assay. (A) Wild-type (atg8Δ pep4Δ CUP1p-Atg8) cells or (B) atg8Δ pep4Δ cells carrying an empty vector were grown to exponential phase in SMD-URA medium. Cells were first converted to spheroplasts and starved in SD(-N) medium containing 1.2 M sorbitol. Spheroplasts were harvested, resuspended in lysis buffer (PS200), and then disrupted. A preclearing step was carry out to remove unbroken cells by centrifuging cell lysates at 300 × g and to obtain total cell lysates (T). To get prApe1-enriched membrane fractions, the total cell lysates were further separated into 5,000 × g suppernatant (S5) and pellet (P5) fractions. The prApe1-containing P5 fractions were split into four aliquots and subjected to different conditions: No treatment, 0.2% Triton X-100 (TX-100), proteinase K (PK), or proteinase K in the presence of 0.2% Triton X-100. The samples were precipitated using 10% TCA, acetone washed twice and subjected to immunoblot analysis using anti-Ape1 antiserum. For internal controls to verify the complete lysis of spheroplasts and proper membrane separation, samples were analyzed using anti-Pgk1 and anti-Ape1 antisera. The integrity of organelle compartments in the P5 fractions were tested by examining the proteolytic cleavage of the precursor form of Prc1. This figure includes data previously published in reference Citation20 and reproduced by permission of Landes Bioscience.