Figures & data
Figure 1. Mutations in epg-9 cause defects in the autophagy pathway. Scale bars (whole embryos: 10 μm; inserts: 5 μm) are only shown once, because C. elegans embryos remain the same size during embryogenesis. (A and B) In wild-type embryos, T12G3.1::GFP is weakly expressed and diffuse in the cytoplasm. (A) Nomarski image of the embryo shown in (B). (C) In epg-9 mutant embryos, T12G3.1::GFP is expressed at greatly elevated levels and accumulates into a large number of aggregates. (D and E) SEPA-1 aggregates are absent in wild-type embryos at the comma stage. (D) DAPI image of the embryo shown in (E). (F) A large number of SEPA-1 aggregates are formed in epg-9 mutant comma stage embryos. Comma stage embryos are shown in (D–F). (G–I) T12G3.1 aggregates (G), detected by anti-T12G3.1 antibody, and PGL granules (H), detected by anti-SEPA-1 antibody, are in close proximity but separable (I) in epg-9 mutant embryos. Inserts: magnified view. A ~200 cell stage embryo is shown in (G–I). (J) Survival of epg-9 mutant L1 larvae is reduced compared with wild type under food depletion conditions (log-rank test, p = 0.000). The median survival duration of wild type and epg-9 mutants was 15.000 [95% confidence interval (CI) 14.920–15.080] and 6.000 (95% CI 5.628–6.372) days, respectively. The difference in survival was compared using the Kaplan–Meier method and log-rank tests.
![Figure 1. Mutations in epg-9 cause defects in the autophagy pathway. Scale bars (whole embryos: 10 μm; inserts: 5 μm) are only shown once, because C. elegans embryos remain the same size during embryogenesis. (A and B) In wild-type embryos, T12G3.1::GFP is weakly expressed and diffuse in the cytoplasm. (A) Nomarski image of the embryo shown in (B). (C) In epg-9 mutant embryos, T12G3.1::GFP is expressed at greatly elevated levels and accumulates into a large number of aggregates. (D and E) SEPA-1 aggregates are absent in wild-type embryos at the comma stage. (D) DAPI image of the embryo shown in (E). (F) A large number of SEPA-1 aggregates are formed in epg-9 mutant comma stage embryos. Comma stage embryos are shown in (D–F). (G–I) T12G3.1 aggregates (G), detected by anti-T12G3.1 antibody, and PGL granules (H), detected by anti-SEPA-1 antibody, are in close proximity but separable (I) in epg-9 mutant embryos. Inserts: magnified view. A ~200 cell stage embryo is shown in (G–I). (J) Survival of epg-9 mutant L1 larvae is reduced compared with wild type under food depletion conditions (log-rank test, p = 0.000). The median survival duration of wild type and epg-9 mutants was 15.000 [95% confidence interval (CI) 14.920–15.080] and 6.000 (95% CI 5.628–6.372) days, respectively. The difference in survival was compared using the Kaplan–Meier method and log-rank tests.](/cms/asset/679fc344-4cd2-4829-a49e-4604bda38220/kaup_a_10921163_f0001.gif)
Figure 2. epg-9 mutants show autophagy phenotypes characteristic of unc-51/Atg1 and epg-1/Atg13 mutants. (A) Western analysis shows the accumulation of both forms of LGG-1-I and LGG-1-II (lipidated form) in epg-9, epg-1 and unc-51 mutants compared with wild-type animals. Actin serves as a loading control. (B and C) LGG-1 forms distinct small punctate structures in a wild-type embryo. (D–G) In epg-9 (D and E), unc-51 (F) and epg-1 (G) mutant embryos, LGG-1 puncta are absent in most cells but forms aggregates in a few cells that are bigger in size and stronger in intensity than those in wild-type embryos. (B and D) DAPI images of the embryos shown in (C and E), respectively. ~200 cell stage embryos are shown in (B–G). (H and I) ATG-9::GFP is diffusely localized in the cytoplasm in a wild-type embryo. (H) DIC image of the embryo shown in (I). (J and K) In unc-51 (J) and epg-9 (K) mutant embryos, ATG-9::GFP accumulates into a large number of punctate structures. Inserts: magnified view. (L) In epg-3 mutant embryos, PGL granules, detected by anti-SEPA-1 antibody, accumulate and form enlarged irregular-shaped clusters. (M) PGL granules are round-shaped and dispersed in the cytoplasm in epg-3; epg-9 double mutants. (N) Immunostaining signals for PGL granules (green), detected by anti-SEPA-1 antibody, and T12G3.1 aggregates (red) are largely overlapping in epg-3 mutant embryos. (O) PGL granules (green) and T12G3.1 aggregates (red) are separable in the epg-3; epg-9 double mutants. (P) LGG-1 puncta are enlarged and accumulate in epg-3 mutant embryos. (Q) LGG-1 puncta are absent in most cells and only accumulate in a few cells in epg-3; epg-9 double mutants. (R) SEPA-1 aggregates (green) and LGG-1 puncta (red) are largely colocalized in epg-3 mutant embryos. (S) SEPA-1 aggregates (green) are separable from the few LGG-1 puncta (red) in epg-3; epg-9 mutant embryos. Separate images for (N, O, R and S) are shown in Figure S2. (T–W) In cup-5 mutants, SEPA-1::GFP accumulates in autolysosomes and the GFP signal is weaker in intensity and the size of the puncta is greater. In cup-5; epg-9 double mutants, distinct SEPA-1 aggregates are formed as in epg-9 single mutants. (T and V) DIC images of the embryos shown in (U and W), respectively.
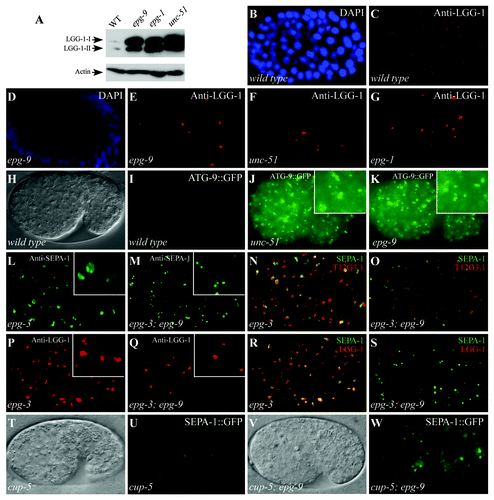
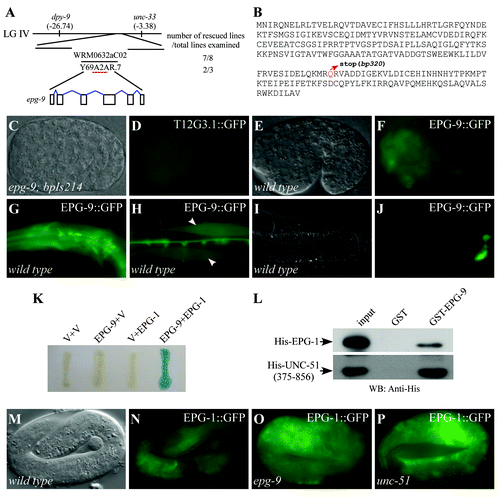
Figure 3. epg-9 encodes a protein with homology to human ATG101. (A) Cloning of epg-9. epg-9 maps on linkage group IV (LG IV). A transgene containing WRM0632aC02 or Y69A2AR.7 rescued defective degradation of T12G3.1 aggregates in epg-9 mutants. Number of rescued and total examined transgenic lines is indicated. (B) Protein sequence of EPG-9. epg-9(bp320) contains a glutamine to stop codon mutation at amino acid 182 (highlighted in red). (C and D) The integrated transgene carrying the translational epg-9 reporter, bpIs214, is functional in rescuing defective degradation of T12G3.1 aggregates in epg-9 mutants. Compared with , no T12G3.1 aggregates accumulate in epg-9; bpIs214 embryos. (C) Nomarski image of the embryo shown in (D). (E and F) epg-9::gfp is diffusely localized in most cells during embryogenesis. (E) Nomarski image of the embryo shown in (F). (G–J) At postembryonic stages, epg-9::gfp is expressed in pharyngeal muscles and neurons in the head region (G), body wall muscles (arrows) (H) and intestinal cells (I and J). (I) Nomarski image of the animal shown in (J). (K) Interaction between EPG-9 and EPG-1 in a yeast two-hybrid assay using an X-gal assay. EPG-9 was cloned into pPC97 and EPG-1 was cloned into pPC86. V stands for the empty pPC97 or pPC86 vector. (L) EPG-9 directly interacts with EPG-1 and UNC-51 in an in vitro pull-down assay. GST-tagged EPG-9 immobilized on glutathione Sepharose beads was incubated with His-tagged EPG-1 or His-tagged UNC-51(375–856). Proteins retained after extensive washes were detected by western analysis using an anti-His antibody. 20% of the protein used for binding serves as input. (M–P) Expression of epg-1::gfp in wild-type (M and N), epg-9 (O) and unc-51 (P) mutants. The EPG-1::GFP signal is slightly stronger in epg-9 and unc-51 mutants. (M) Nomarski image of the embryo shown in (N).