Figures & data
Figure 1. The protective effect of PrPC against H2O2-induced cell death. (A) The expression of PrPC in distinct stable cell lines and the embryo hippocampus of ICR mice. Western blot analysis was conducted using anti-PrP (3F10) in lysates from Zürich I Prnp0/0 hippocampal neuronal cell lines transfected with murine Prnp (Prnp+/+) or with an empty vector (Prnp0/0), several hippocampi from 14-d-old embryos of ICR mice and 3 different WT hippocampal neuronal cell lines of ICR mice (ZW 13–2, ZW 13–2 and ZW 13–3). The expression of PrPC was quantified using densitometry analysis (right panel). (B and C) Morphological appearance of Prnp+/+ and Prnp0/0 cells as observed under an inverted microscope. In (B and D), Prnp+/+ and Prnp0/0 cells were incubated with the indicated concentrations of H2O2 for 6 h. In (C and E), cells were treated with 500 μM H2O2 for the indicated time periods. (D and E) Cell viability was measured using the WST-1-based assay. The data are presented as the means ± standard deviation (S.D.) of 3 independent experiments performed in triplicate. *p < 0.02 and **p < 0.001, significant differences between treated and untreated cells; †p < 0.01 and ‡p < 0.001, significant differences between Prnp+/+ and Prnp0/0 cells.
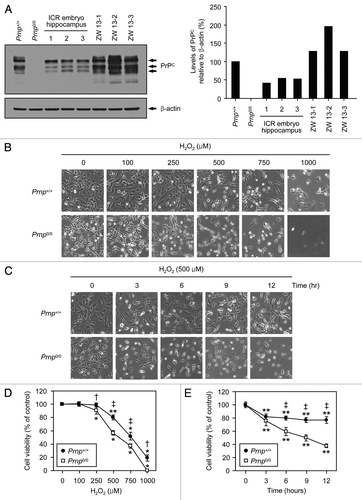
Figure 2. The induction of autophagy in Prnp+/+ and Prnp0/0 cells exposed to H2O2. (A) Cells were incubated with the indicated concentrations of H2O2 for 6 h. (B) Cells were treated with 500 μM H2O2 for the indicated time periods. Cell lysates were subjected to western blot analysis using anti-LC3B or anti-β-actin antibodies (A and B), and the expression of LC3B-II was quantified using densitometry analysis (right panels). At least 3 independent experiments were used to assess the percentage of LC3B-II expression relative to that of β-actin. (C) Immunofluorescence microscopy analysis using anti-LC3B antibody and DAPI counterstaining in both Prnp+/+ and Prnp0/0 cells treated with 500 μM H2O2 for 3 h. The average number of LC3B puncta per cell is shown in the lower panel. At least 60 cells in several fields were counted in each experiment. The data represent the means ± SD of 3 independent experiments. *p < 0.05 and **p < 0.01, significant differences between H2O2-treated and untreated cells; †p < 0.05 and ‡p < 0.01, significant differences between Prnp+/+ and Prnp0/0 cells. Scale bar: 20 µm.
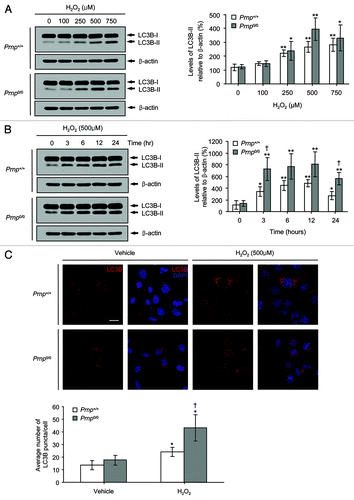
Figure 3. Impaired autophagic flux in Prnp0/0 cells exposed to H2O2. Cells were incubated with or without 500 μM H2O2 in the presence or absence of 20 nM bafilomycin A1 (Baf A1) for the indicated time periods. Cell lysates were subjected to western blot analysis using anti-LC3B or anti-β-actin antibody (upper panel), and the expression of LC3B-II was quantified using densitometry analysis (lower panel). At least 3 independent experiments were used to assess the percentage of LC3B-II expression relative to that of β-actin. The data represent the means ± SD of 3 independent experiments. *p < 0.05 and **p < 0.01, significant differences between Baf A1-treated and untreated cells; †p < 0.05 and ‡p < 0.01, significant differences between H2O2-treated and untreated cells.
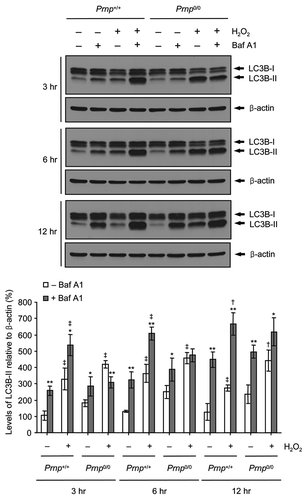
Figure 4. Apoptosis activation in Prnp+/+ and Prnp0/0 cells exposed to H2O2. (A) Cells were incubated with the indicated concentrations of H2O2 for 6 h. (B) Cells were treated with 500 μM H2O2 for the indicated time periods. Cell lysates were subjected to western blot analysis with anti- CASP3 or anti-β-actin antibodies (A and B), and the expression of cleaved CASP3 was quantified using densitometry analysis (right panels). At least 3 independent experiments were used to assess the percentages of cleaved CASP3 expression relative to that of β-actin. †p < 0.05, significant differences between Prnp+/+ and Prnp0/0 cells. (C) Cells were preincubated with the indicated concentrations of zVAD-fmk for 3 h and treated with or without 500 μM H2O2 for 6 h. Cell viability was determined using the WST-1 reagent assay. The data are represented as the percent viability normalized to mock-treated control groups (100%). The data are presented as the means ± SD of 3 independent experiments performed in triplicate. (D) Cells were treated with 500 μM H2O2 for the indicated time periods and fractionated into cytoplasmic and nuclear fractions. Each fraction was analyzed by western blotting with anti-AIFM1, anti-ENDOG, anti-ENO1 (cytosolic fraction markers) and anti-HDAC2 antibody (nuclear fraction marker).
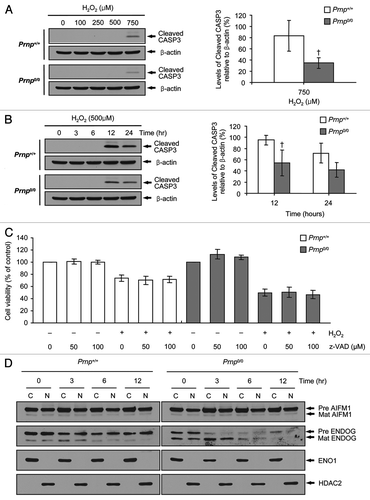
Figure 5. Effects of the autophagy inhibitor 3-MA and the autophagy inducers rapamycin (Rapa) and trehalose (Treha) on H2O2-induced cell death. (A–F) Prnp+/+ and Prnp0/0 cells were pretreated with or without 5 mM 3-MA, 10 nM Rapa or 100 mM Treha for 1 h (Rapa) or 3 h (3-MA and Treha) and with or without 500 μM H2O2 for 6 h. (A, C and E) Cell lysates were subjected to western blot analysis using an anti-LC3B, antibody in PathScan® Multiplex Western Cocktail I to detect phosphorylated AKT1 or RPS6 or anti-β-actin antibody. (B, D and F) Cell viability was determined using the WST-1 reagent assay. The data are represented as the percent viability normalized to mock-treated control groups (100%). The data are presented as the means ± SD of 3 independent experiments performed in triplicate.
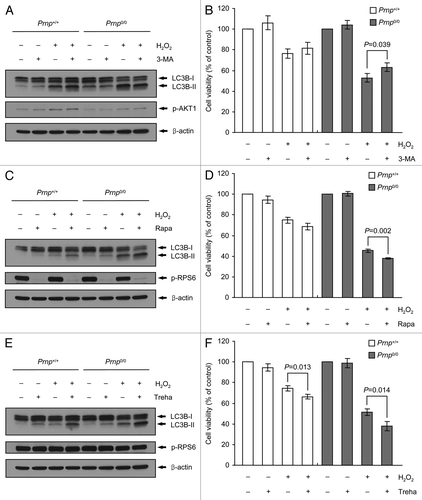
Figure 6. Augmentation of H2O2-induced cell death using Atg7 knockdown in Prnp+/+ cells. (A and B) Prnp+/+ and Prnp0/0 cells were transfected with nontargeting (NT) or Atg7 siRNA, respectively. The siRNA-transfected cells were subsequently treated with or without 500 μM H2O2 for 6 h. (A) Cell lysates were subjected to western blot analysis using anti-ATG7, anti-LC3B or anti-β-actin antibody. (B) Cell viability was determined using the WST-1 reagent assay. The data are represented as the percent viability normalized to H2O2-untreated control groups (100%). The data are presented as the means ± SD of 3 independent experiments performed in triplicate.
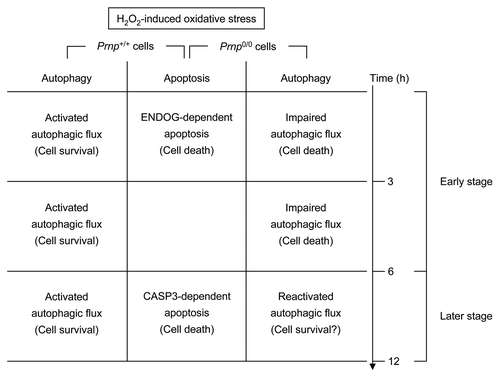
Figure 7. Schematic representation of time-dependent H2O2-induced cell death in Prnp+/+ and Prnp0/0 cells. In 2 types of cells, caspase-independent and caspase-dependent apoptosis were observed through ENDOG and cleaved CASP3, respectively. Activated autophagic flux protects against oxidative stress in Prnp+/+ cells, while impaired autophagic flux plays a role in neuronal cell death in Prnp0/0 cells. This impaired autophagic flux is rescued at a later time point (12 h), suggesting that the reactivated or restored autophagic flux may play an additional protective role.