Figures & data
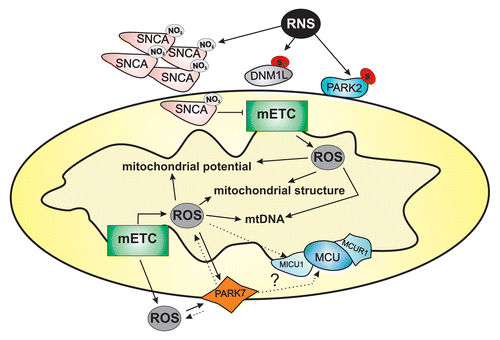
Figure 1. Mitochondrial integrity is a key factor involved in mitophagy, which is regulated by specific fission proteins, including DNM1L, FIS1, and MFF, which are recruited by mitophagy-related inducers. The figure shows the involvement of the mitochondrial Ca2+-sensing protein RHOT1, an OMM protein that under resting conditions interacts with the cytoplasmic adaptor protein TRAK2, mediating mitochondrial sliding along the cytoskeleton. Sustained [Ca2+]c elevations induce the RHOT1-dependent motility block, allowing DNM1L-induced fragmentation. Contact sites have been recently shown to be required for the DNM1L polymerization process that generates a constriction around mitochondria, a process that is necessary for mitochondrial fission. In depolarized neurons, PINK accumulation favors RHOT1 phosphorylation, inducing PINK-RHOT1-PARK2 aggregation. This complex promotes RHOT1 degradation, preventing mitochondrial movement and quarantining the damaged mitochondria.
![Figure 1. Mitochondrial integrity is a key factor involved in mitophagy, which is regulated by specific fission proteins, including DNM1L, FIS1, and MFF, which are recruited by mitophagy-related inducers. The figure shows the involvement of the mitochondrial Ca2+-sensing protein RHOT1, an OMM protein that under resting conditions interacts with the cytoplasmic adaptor protein TRAK2, mediating mitochondrial sliding along the cytoskeleton. Sustained [Ca2+]c elevations induce the RHOT1-dependent motility block, allowing DNM1L-induced fragmentation. Contact sites have been recently shown to be required for the DNM1L polymerization process that generates a constriction around mitochondria, a process that is necessary for mitochondrial fission. In depolarized neurons, PINK accumulation favors RHOT1 phosphorylation, inducing PINK-RHOT1-PARK2 aggregation. This complex promotes RHOT1 degradation, preventing mitochondrial movement and quarantining the damaged mitochondria.](/cms/asset/4b3198c8-6b3f-4905-adfc-2ce0d611eac8/kaup_a_10924795_f0001.gif)
Figure 2. The ubiquitination of a subset of OMM proteins including VDAC1, TOMM, and MFNs by the ubiquitin ligase PARK2 promotes the dissipation of Ψm, inducing the recruitment of a series of adaptor proteins (such as SQSTM1) that promote mitochondrial sequestering. Ψm represents a huge driving force for Ca2+-entry into the organelle through the recently identified mitochondrial calcium uniporter MCU. The activity of the channel is regulated by the mitochondrial proteins MICU1/mitochondrial calcium uptake 1 and MCUR1/mitochondrial calcium uniporter regulator 1. The loss of Ψm impairs Ca2+ uptake and mitochondrial Ca2+-buffering causing PINK1 accumulation and stabilization on the OMM. In the inset, Pink1 KO cells show reduced Ψm via the increased mitochondrial Ca2+ uptake and mPTP (mitochondrial permeability transition pore) opening.
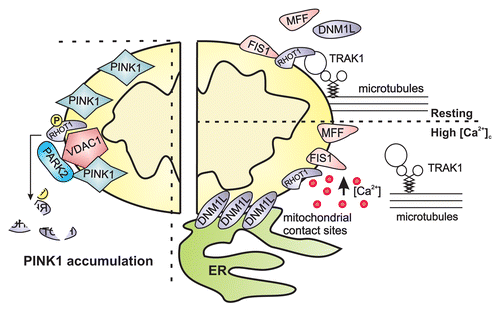
Figure 3. ROS and RNS are responsible for the decrease in mitochondrial potential, mitochondrial fission and mtDNA mutations. Indeed, ROS and RNS regulate several mitophagic molecular targets through protein modification. ROS, reactive oxygen species; RNS, reactive nitrogen species; mETC, mitochondrial electron transport chain complex.