Figures & data
Figure 1. Trehalose treatment prolongs the life span of mutant SOD1 transgenic mice. (A) SOD1G86R transgenic mice were intraperitoneally injected with 2 g/Kg of trehalose (n = 17), 2 g/Kg of glucose (n = 15) or an equivalent volume of PBS (untreated, n = 15) starting at 35 d of age. The compounds were also permanently provided in a concentration of 3% in the drinking water (free consumption). Mouse survival was monitored over time. Animals presented in (A) were divided into groups of male (B) and female (C) mice. Total group numbers for male mice: trehalose (n = 9), glucose (n = 7) and untreated (n = 8). Females: trehalose (n = 8), glucose (n = 8) and untreated (n = 7). (D) Average life span of animals treated with trehalose, glucose or PBS injected is presented. Survival curves were estimated by Kaplan-Meier statistic analysis. In (A–C), the p value was calculated with log-rank test (Mantel-Cox) to compare trehalose with PBS treated animals.
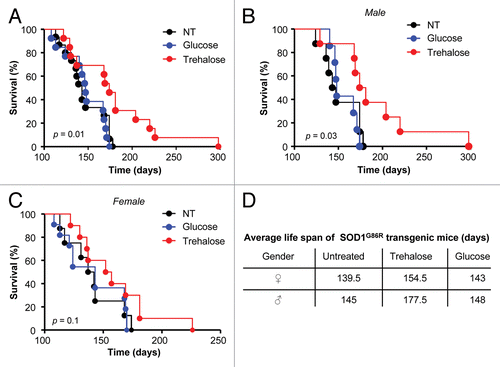
Figure 2. Trehalose treatment ameliorates the progression of disease signs in mutant SOD1 transgenic mice. (A) Disease onset was monitored in SOD1G86R animals described in after treatment with trehalose, glucose or PBS. Disease onset was calculated as the day when animals lost 5% of their total body weight. (B) In parallel, rotarod performance was monitored as described in Materials and Methods to determine disease onset. (C) The appearance of several disease signs was assessed to obtain a disease score and evaluate the progression of the severity of the disease. A minimum value of 3 was used to define disease onset. Scored parameters included the appearance of spinal curvature, signs of paralysis, slight hind limbs tremor, ruffling fur and falling of hind limbs (see Fig. S1A and S1B). In (A–C) no statistically significant differences were determined between the experimental groups using Student’s t-test. (D) Average disease progression of mutant SOD1 transgenic animals treated with trehalose, glucose and untreated, as indicated, by visual observation of disease signs. Number of animals used per group: trehalose (n = 7, male; n = 5 female), glucose (n = 6, male; n = 5 female) or an equivalent volume of PBS (n = 4, male; n = 3 female). Statistical analysis of disease duration is presented in Figure S1C.
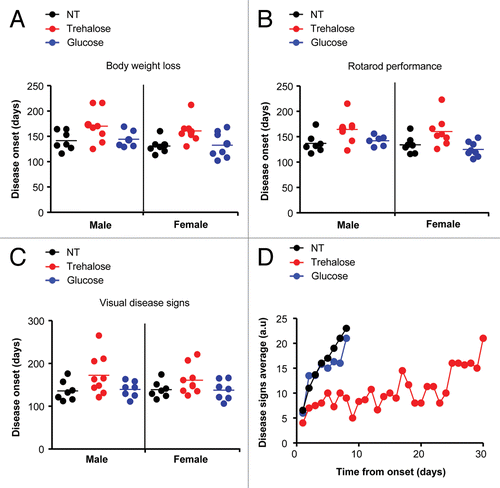
Figure 3. Trehalose administration decreases mutant SOD1 aggregation, reduces astrogliosis and improves neuronal survival. (A) SOD1 aggregation was determined in spinal cord protein extracts derived from SOD1G86R transgenic mice treated with trehalose, glucose or PBS. Each track represents one independent animal. Tissue was collected at the symptomatic phase of the disease. HSP90 was monitored as a loading control. Image was assembled from cropped lanes of the same western blot analysis. (B) Quantification mutant SOD1 aggregation levels in spinal cord extracts normalized with the loading control from (A) and Figure S3A. Number of animals analyzed per treatment in male and female mice respectively: Trehalose (n = 8 and 7), glucose (n = 7 and 6) and PBS (n = 8 and 8). (C) Immunofluorescence analysis of GFAP staining in spinal cord tissue derived from animals described in at the symptomatic stage of the disease. Upper panel: A representative image is shown of the analysis of four independent animals. A zoom is presented at the left of the selected area on a rectangle. (Scale bar: 250 μm and 60 μm). Bottom panel: quantification of GFAP signal is shown (see methods). (D) Motoneuron survival was determined in the ventral horn of the spinal cord after toluidine blue staining. Motoneurons were identified by their distribution, morphology and size. Tissue was collected in animals at 120 d of age. Littermate control mice were used for the analysis. In all panels with statistical analysis mean and standard deviation (B) or mean is presented with a line (C and D). p values were calculated with Student’s t-test, *p < 0.01; **p < 0.005; ***p < 0.0005. n.s.: non-significant.
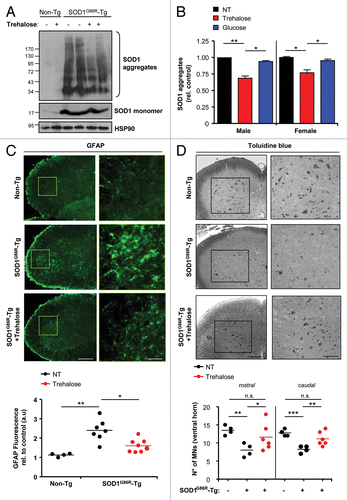
Figure 4. Treatment of mutant SOD1 transgenic mice with trehalose induces autophagy in spinal cord motoneurons. (A) LC3 expression was determined in spinal cord samples derived from SOD1G86R transgenic or nontransgenic mice (Non-Tg) treated with trehalose, glucose, or PBS. Tissue was collected at the symptomatic phase of the disease. HSP90 levels were monitored as loading control. Each well represents an independent animal. (B) In parallel, SQSTM1 levels were monitored by western blot. The relative expression (Rel. Ex.) level in each well was quantified and normalized to HSP90 and presented as a fold induction in comparison with the expression observed in nontransgenic untreated animals. (C) LC3 distribution was assessed by indirect immunofluorescence (green) in motoneurons of animals treated as described in . Motoneurons were identified after staining with RBFOX3/NeuN (red), in combination with the identification of their morphology, size and distribution (left panel). Right panel: The percentage of RBFOX3/NeuN-positive neurons containing LC3 positive dots (> 3 puncta per cell) was quantified. Mean is presented with a line. p values were calculated with Student’s t-test, *p < 0.01 (Scale bar: 20 μm). (D) Phosphorylation of MTOR was determined by western blot in samples presented in (A). Total MTOR and HSP90 were analyzed as control. As positive control, NSC34 motoneuron cells were treated with EBSS to induce nutrient starvation and analyzed in the same gel.
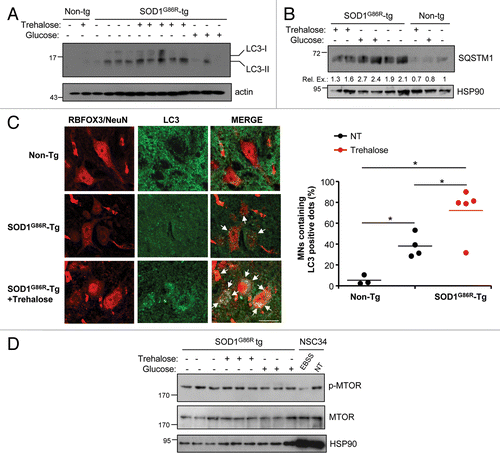
Figure 5. Trehalose modulates the expression of a cluster of autophagy-regulatory genes and the activation of FOXO1. (A) Spinal cord tissue from mutant SOD1 transgenic mice treated with trehalose, glucose or PBS was collected at the symptomatic phase of the disease and relative mRNA levels of indicated genes was analyzed by real-time PCR using total cDNA. As control, non-transgenic animals were also analyzed. Male and female mice were analyzed. Values were normalized with β-actin (Actb) levels. Mean is presented with a line. (B) Right panel: The subcellular distribution of FOXO1 was assessed by immunofluorescence analysis (green) of spinal cord tissue of animals described in (B) (Scale bar: 40 μm). Right panel: The number of cells presenting nuclear localization of FOXO1 was quantified per area in the ventral horn of the spinal cord. Littermate controls were used for the analysis. Mean is presented with a line. In (A–C), p values were calculated with Student’s t-test, *p < 0.01; **p < 0.005. n.s.: nonsignificant.
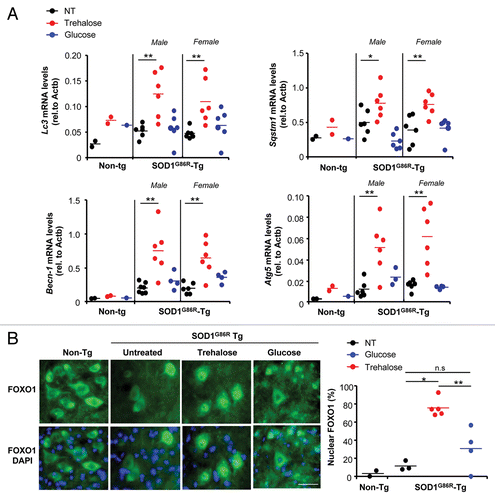
Figure 6. Trehalose triggers autophagy-mediated degradation of mutant SOD1 in NSC34 motoneuron cells. (A) NSC34 cells were transiently transfected with an expression vector for human SOD1G85R fused to EGFP. Cells were cotreated for 48 h or preincubated 24 h before transfection with 100 mM trehalose. SOD1 aggregates were visualized by western blot analysis. Each well represents an independent experiment. SOD1G85R monomers are also indicated. Bottom panels: The levels of LC3 and HSP90 (loading control) were monitored in the same samples. (B) Immunofluorescence analysis of endogenous LC3 (red) and SOD1G85R-EGFP (green) using spinning disk microscopy. As control, NSC34 cells were transiently transfected with SOD1WT-EGFP (upper panel). Lower panel: The number of cells containing LC3-positive dots was quantified. Mean and standard deviation is presented. Scale bar: 20 μm. (C) NSC34 cells were transiently transfected with an expression vector for SOD1G85R-EGFP and treated with or without 100 mM trehalose. In addition, cells were exposed to a cocktail of lysosomal inhibitors (200 nM bafilomycin A1, 10 μg/ml pepstatin, 10 μg/ml E64d) or 10 mM 3-methyladenine (3-MA). Then, the levels of mutant SOD1 aggregation and the monomeric form were determined by western blot. As control for the treatments, LC3 expression was determined. (D) To monitor autophagy fluxes in the absence of inhibitors, NSC34 cells were transiently transfected with an expression vector for mCherry-GFP-LC3 and treated with or without 100 mM trehalose. Then, the presence of GFP-mCherry- (i.e., autophagosomes) and mCherry-positive (i.e., autolysosomes) dots were visualized using spinning disk microscopy. Right panel: quantification of the ratio between red vs. yellow dots to assess an autophagy flux index. Mean and standard error is presented. p values was calculated with Student’s t-test, *p < 0.01; **p < 0.005; ***p < 0.0005.
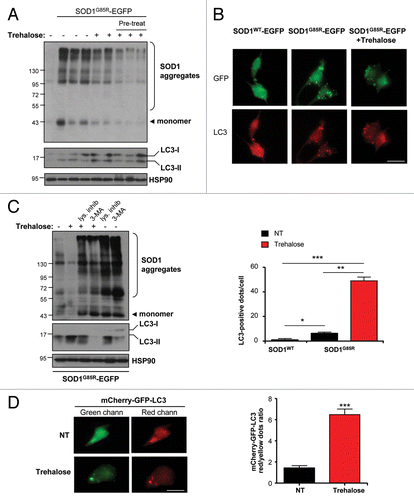
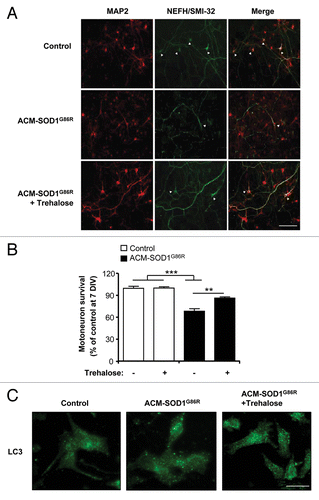
Figure 7. Trehalose reduces the death of primary motoneurons against conditioned media from mutant SOD1 transgenic astrocytes. (A) Cell culture media was conditioned for 7 d (from 2 to 3 weeks) by astrocytes derived from transgenic mice overexpressing SOD1G86R (ACM-SOD1G86R). Primary rat spinal cord cultures (3 DIV) were exposed to ACM-SOD1G86R for 4 d alone or with trehalose (175 mM) and fixed at 7 DIV to assay cell survival with immunocytochemistry. Cultures were fixed at 7 DIV and double-labeled with anti-MAP2 antibody (red) to visualize interneurons and motoneurons and with the anti-NEFH/SMI-32 antibody (green) to identify motoneurons (arrow). Scale bar: 200 µm. (B) The percentage of motoneuron survival was quantified in experiments presented in (A). Values represent means and standard error from three independent experiments performed in duplicate. ***p < 0.001 relative to control media in the absence or presence of trehalose (white bars) and analyzed by one-tailed t-test followed by a Welch post hoc test. **p < 0.007 relative to ACM-SOD1G86R with trehalose (black bar) and analyzed by Student’s t-test. (C) LC3 distribution was analyzed by immunofluorescence in experiments presented in (A).