Figures & data
Figure 1. MAPK1/3 inactivation in unfertilized eggs triggers cycles of NEB and egg constrictions linked to Cai variations that precede egg fragmentation. (A) Morphology of one egg treated with 5 µM U0126. (i) DIC images. Time (bottom left) is given in min after U0126 addition (t = 0). (ii) Time course of nuclear appearance (dark gray) and contractions occurrence (light gray). 0 and 1 are arbitrary values indicating absence of both nucleus and constriction (round cell, given value = 0) and clear nucleus or deep constriction (given value = 1). (B) Correlation between constrictions and Cai changes. (i) Image sequence (see Vid. S1) starting at time 320 min after U0126 addition. Time between 2 images shown is 4 min. (ii) Variations of Cai levels in the egg shown in (Bi). Sequences of contraction (indicated by arrows)/relaxation were determined as in (A). (iii) Comparison between a common fertilization Cai signal recorded after sperm addition (Fert) and an example of Cai bursts occurring between 340 and 550 min after addition of 5 µM U0126.
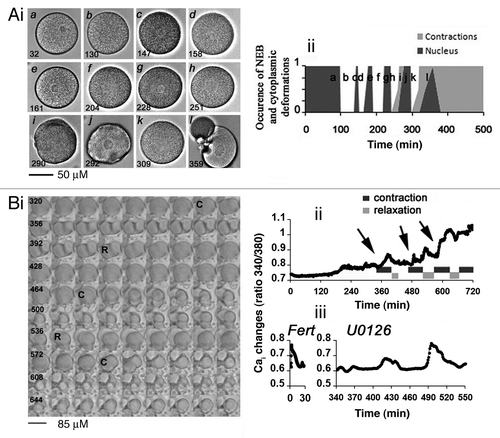
Table 1. Analysis of NEB induced by MAP2K/MEK inhibition
Table 2. Analysis of contraction cycles induced by MAP2K/MEK inhibition
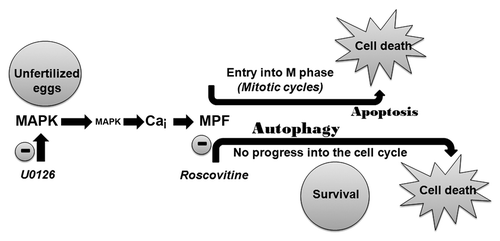
Figure 2. Inactivation of MAPK1/3 triggers a Ca-dependent apoptotic pathway with stimulation of CASP3. (A) EGTA buffer injection delays egg fragmentation induced by 5 µM U0126. (i) Images chosen at representative times (min noted in white) taken from a movie of 2 eggs treated with U0126 and injected with or without EGTA buffer (white asterisk). (ii) Time course of fragmentation after injection (7 eggs) or no injection (10 eggs) of EGTA in eggs from the same population treated with 5 µM U0126. Two experiments gave similar results, one is shown. (B) Time course of CASP3 activity after treatment of unfertilized eggs with 5 µM U0126. Representative images of eggs at the indicated time points are displayed around the graph.
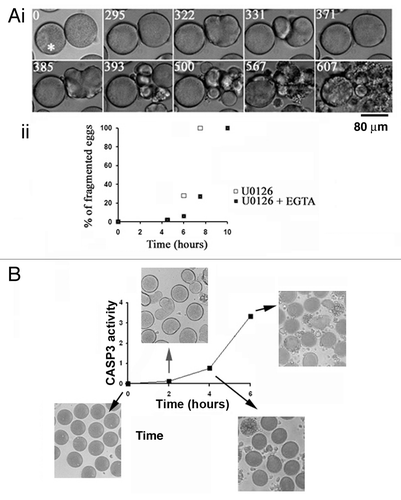
Figure 3. Roscovitine delays fragmentation of unfertilized eggs whether or not they are deprived of MAPK1/3 activity. Unfertilized eggs were not treated (Control, C) or treated with 2 µM U0126 (U), 20 µM roscovitine (R), or with both inhibitors (UR). (A) Fragmentation. (i) time course in two different batches of eggs (1 and 2); (ii) images from experiment 1 of unfertilized eggs taken at time 0, 10 or 14 h after treatment. Only eggs showing clear huge blebs and extrusions of cytoplasm were counted as fragmented (indicated with a star in ii). (Bi) P-MAPK1/3 levels; (ii) P-CDK1(Tyr15) levels. Western blot (left panels) using anti-P-MAPK1/3 antibody or anti-P-CDK1(Tyr15) antibody and quantification of signals (right panels).
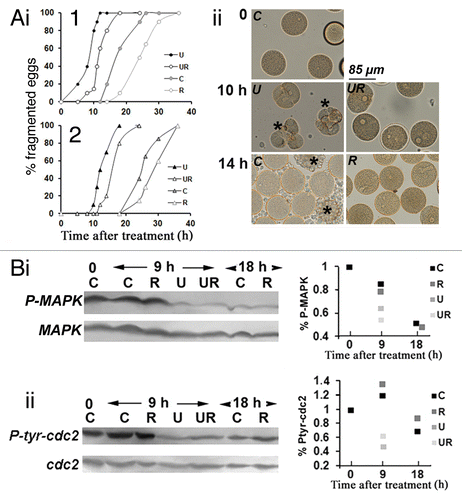
Figure 4. Roscovitine does not inhibit Cai changes induced by MAPK1/3 inactivation in unfertilized eggs. (A) Image sequence from 505 to 710 min showing changes in the morphology of a representative egg injected with fura-2 and treated with 5 µM U0126 and 20 µM roscovitine. Time between 2 images is 5 min (time displayed in black). This egg showed movements of the nucleus which remained until at least 610 min (arrows) and kept its round shape until 575 min. (B) Changes in Cai in the egg shown in (A).
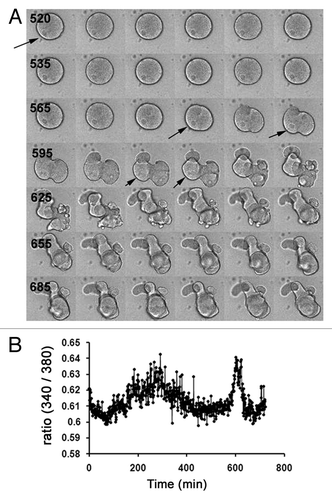
Figure 5. Survival of eggs deprived of MAPK1/3 and CDK activities involves LC3B. (A) LC3B cleavage. (i) Western blot analysis with anti-LC3B Ab (upper panel) and internal control with anti-TUBA (lower panel) with quantification of bands (histograms). Eggs were not treated (control eggs, C) or treated with staurosporine (St), U0126 (U) or roscovitine (R) only or with both U0126 and roscovitine (UR) for 7, 10, 12 or 20 h. The lower bands of the gel (LC3B-II) and both bands (total LC3B) have been quantified as explained in Materials and Methods. (ii) Changes in the proportion of LC3B-II. Three experiments that gave similar results were pooled. Percentage of LC3B-II (lower band) as visualized in (i) is here expressed relative to the total amount of LC3B arbitrarily taken as 100. Signals are obtained in control eggs (C, time zero), eggs treated during 10 to 13 h with U0126 only (U), or eggs treated during 17 to 20 h with roscovitine only (R) or with U0126 and roscovitine (UR). In the three experiments, eggs treated with U0126 only died within 16 h treatment. Only values (means ± sem) obtained in UR eggs are significantly different from those of C eggs (Student test, P < 0.01). (B) LC3B cytoplasmic distribution. (i) Observation of eggs treated for 10 h with U0126 only (U) or with U0126 and roscovitine for 18 h (UR) after fixation and immunolabeling with the anti-LC3B Ab. An image by transmitted light microscopy (left panels) and a z projection of five images taken from a stack of images obtained by confocal microscopy and localized in the center of the egg (U) or around the nucleus (UR) (right panels) are shown. An enriched particulate distribution of LC3B (arrow) around the nucleus (star) was only seen in UR eggs. (ii) Variation in the amount of large LC3B structures. Particles larger than 2.95 µm2 were quantified as explained in Materials and Methods. Values (means ± sem, 10 eggs/condition) obtained UR eggs are significantly different from those of C eggs (Student test, P < 0.01). (C) Effect of injection (arrow) of the anti-LC3B antibody before treatment of unfertilized eggs with U0126 only (i) or with both U0126 and roscovitine (ii).
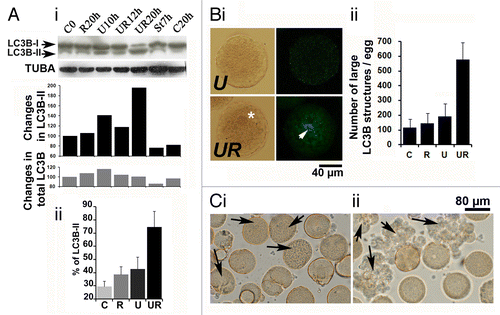
Figure 6. Roscovitine increases the amount of AVOs in U0126-treated eggs. (A) Visualization of AVOs by confocal microscopy. C, R, U, and UR are as described in . A z projection of five images taken from a stack of images and localized in the center of the egg (U) or around the nucleus (C, R, and UR) are shown. AO accumulates in eggs as seen by the green color detected in the three conditions (left panels), and fluoresces in red (right panels) as an indication of acidity. Note that AO enters the large nucleus of UR eggs but without showing high intensity of red fluorescence. (B) Quantification of AVOs. (i) Global fluorescence; (ii) amount of AVO patches of size smaller (S) or larger (L) than 2.95 µm2. Values (means ± sem, 12 eggs/condition) are significantly different (Student t-test, *P < 0.05 or **P < 0.01) or not significantly different (ns) from those of control eggs.
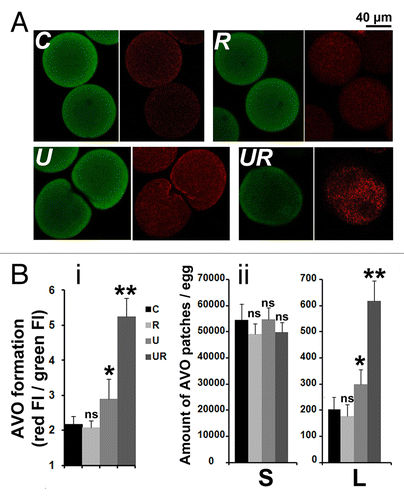
Figure 7. Roscovitine leads to the formation of autophagic vacuoles in U0126-treated eggs. (A) EM observation. C, R, U and UR are as described in . Eggs were either untreated (0) or treated for 4 h or 7 h. Cytoplasm of eggs treated for 7 h with U0126 and roscovitine (UR) retained the general aspect seen in control eggs with visible intracellular compartments including mitochondria (M), vitellin platelets (V) or cortical granules (CG). On the contrary, U0126 treated eggs showed elevation of a fertilization membrane (FM) after 4 h and no clear intracellular structure was visible 7 h after treatment. Eggs treated with roscovitine only appeared as per the control eggs (not shown). (B) Observation at higher magnification of eggs treated for 7 h with U0126 and roscovitine. (i) Five examples (1 to 5) of membrane-enclosed structures that resemble autophagosomes. (ii) Various areas containing ER that appeared very dilated (1 to 2) or containing undilated ER (3) or Golgi (4).
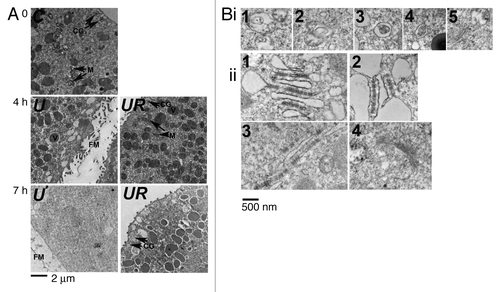
Figure 8. LC3B is detected by immunogold labeling in eggs treated with U0126 and roscovitine for 7 h. (Examples 1 to 7) of images obtained after observation under EM are shown. Immunogold particles (arrow) were always detected close to vesicular structures that were associated together (3, 5, 7) and often localized in the vicinity of ER (1). Some particles were clearly associated with membranes of these structures (stars).
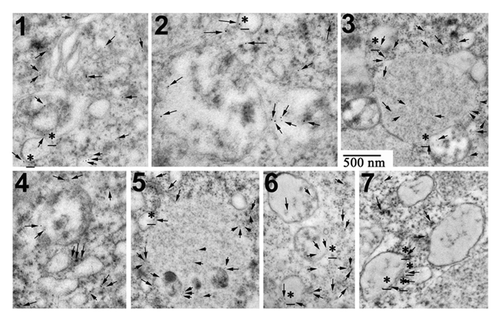
Figure 9. Leupeptin and pepstatin enhance survival of eggs. Light microscopy images of control eggs (C) or eggs treated with 20 µM leupeptin and 7 µM pepstatin (L), 5 µM U0126 (U), 5 µM U0126 and 20 µM roscovitine (UR), U0126 and leupeptin (UL) or with U0126, leupeptin and roscovitin (URL). Eggs were observed 24 h after addition of the drugs. Although all eggs had fragmented without leupeptin and pepstatin, these drugs led to a marked increase in the survival of eggs treated with or without U0126 and/or roscovitine. Note the presence of dark eggs (stars) in the presence of leupeptin and pepstatin.
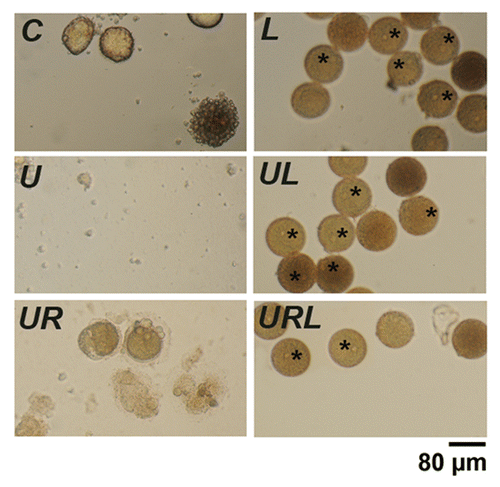
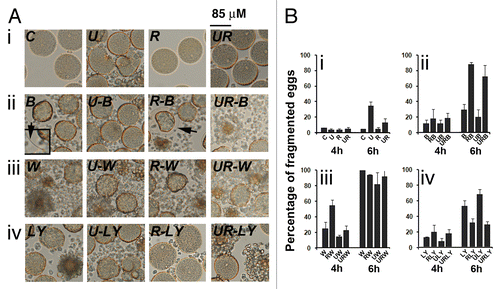
Figure 10. Bafilomycin A1 (Baf) and PtdIns 3-kinase inhibitors act on egg survival. (A) Light microscopy images of control eggs (C) and of eggs treated for 6 h with U0126 (U), roscovitine (R), Baf (B), wortmannin (W), LY294002 (LY), or different combinations of these inhibitors. (B) Assessment of fragmentation percentages measured after 4 h and 6 h treatment. Effect of U0126 and roscovitine treatment (i), Baf (ii), wortmannin (iii) or LY294002 (iv) addition. Activation of the eggs with elevation of a fertilization envelope (inset, 2× magnification) can be seen in Baf-treated eggs (ii).