Figures & data
Figure 1. Generation of HCC cell lines with stable autophagy inhibition via lentivirus-mediated knockdown of BECN1 and ATG5. (A and B) Quantitative real-time PCR and western blot analysis of BECN1 and ATG5 knockdown. Effective shRNAs targeting BECN1 and ATG5 were selected and constructed into 2 lentiviral vectors. The knockdown efficiency of the cotransfection was analyzed by western blot. (C and D) HCC cell lines with stable autophagy inhibition (HCCLM3-ATGi and RFP-expressing HCCLM3-R-ATGi). GFP-LC3 transfection analysis and western blot analysis showed that rapamycin (Rapa) treatment did not induce autophagy in HCCLM3-ATGi or HCCLM3-R-ATGi cells while it induced intense autophagy in HCCLM3 and HCCLM3-R cells without autophagy inhibition.
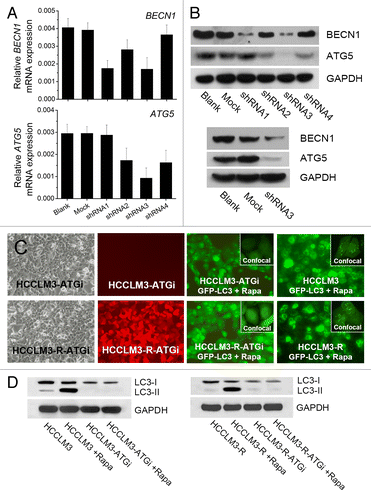
Figure 2. In vivo analysis of the effect of autophagy inhibition on HCC metastasis. (A) Nude mouse model of pulmonary metastasis was established using HCC cells with or without autophagy inhibition (HCCLM3-ATGi or HCCLM3/HCCLM3-Mock). Histopathological analysis showed that there were significantly fewer lung metastases of mice receiving HCCLM3-ATGi cells than in mice subjected to HCCLM3 and HCCLM3-Mock cells. *P = 0.001, **P = 0.044. (From left to right) Mice receiving orthotopic implantation of HCCLM3 / HCCLM3-ATGi / HCCLM3-Mock cells, lung and liver of the mice, representative panoramic image of lung section, lung metastasis (100×) and lung metastasis (200×); (B) Small-animal imaging analysis using RFP-expressing HCCLM3-R-ATGi and HCCLM3-R cells confirmed the histopathological analysis. The images of fluorescence signals were pseudocolored (pink, least intense; red, most intense), and the images of lung metastases and primary HCC tumors were merged onto the corresponding X-ray images.
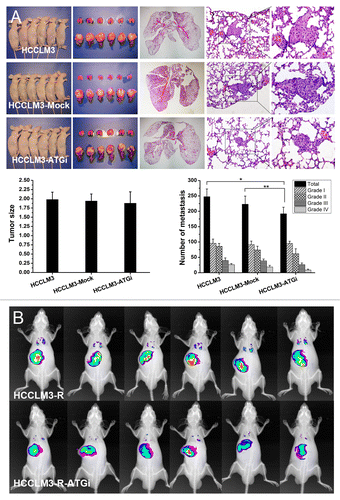
Figure 3. Effect of autophagy inhibition on migration and invasion of HCC cells. (A) Transwell migration and invasion assays showed no effect of autophagy inhibition on the migration and invasiveness of HCCLM3 cells. (B) The scratch assay showed results similar to the transwell migration assay.
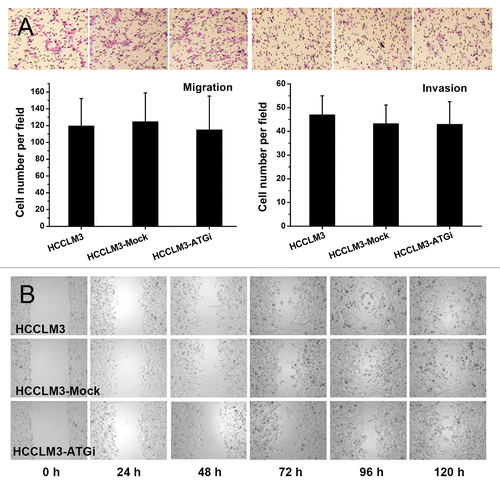
Figure 4. Autophagy inhibition attenuates anoikis resistance of HCC cells via regulation of apoptotic signaling. (A) The in vitro anoikis assay indicated that autophagy inhibition significantly increased the anoikis rate of HCCLM3 cells after cell detachment. (B) In vivo anoikis assays showed that no mice receiving HCCLM3 cells with autophagy inhibition (HCCLM3-ATGi) formed peritoneal metastases and ascites while mice subjected to HCCLM3 cells without autophagy inhibition (HCCLM3) could establish. The HCCLM3 ascites cells were determined by smear test (ST) and flow cytometric DNA analysis (FCM).
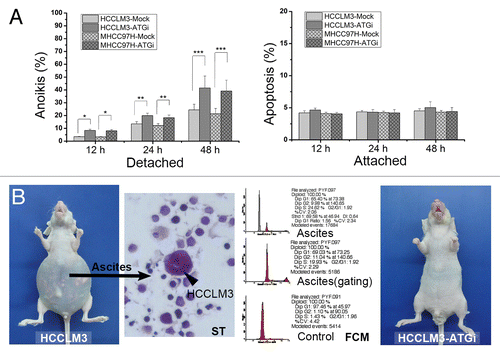
Figure 5. Effect of autophagy inhibition on epithelial mesenchymal transition (EMT) of HCC cells. Western blot analysis of EMT markers expressions (CDH1, VIM, ACTA2, ZEB1, SNAI1, and TWIST1) in HCCLM3 cells with or without autophagy inhibition (HCCLM3 or HCCLM3-ATGi) revealed no significant differences between HCCLM3 and HCCLM3-ATGi cells. Cell lysates from HCCLM3-ATGi and HCCLM3 cells were immunoblotted with indicated antibodies (left). Protein levels were determined by densitometry measurements and normalized (right).
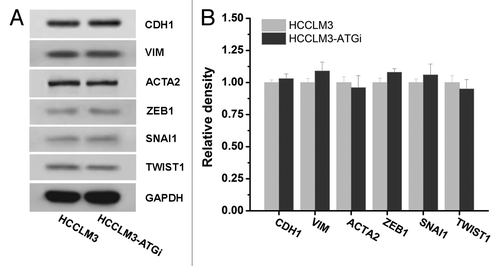
Figure 6. Autophagy inhibition suppresses lung colonization of HCC cells and ER stress is not involved in. (A) Lung colonization assays indicated that autophagy inhibition significantly suppressed lung colonization of HCCLM3 cells (*P = 0.018, **P = 0.040). (From left to right) representative panoramic image of lung section, lung metastasis (100×), and lung metastasis (200×). (B and C) ER stress was induced in metastatic colonies but the suppressive effect of autophagy inhibition on metastatic colonization was not associated with ER stress. Immunohistochemical analysis of ER stress marker HSPA5 expression in lung metastasis and paired primary tumor showed significantly higher HSPA5 expression in lung metastases, indicating that ER stress was induced in metastatic colonization (B). Further in vivo metastasis assays showed that inhibition of ER stress by PBA did not affect lung metastasis (C). The number of lung metastases of mice receiving PBA (PBA group) was not significantly different from those of mice subjected to PBA vehicle (Vehicle group) or no treatment (Blank group). (PBA vs. Blank, P = 0.247; PBA vs. Vehicle, P = 0.136).
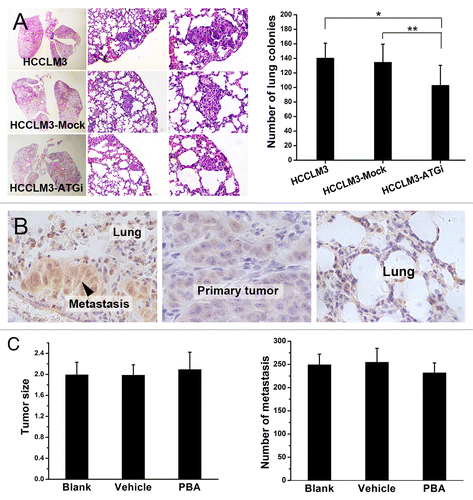
Figure 7. Construction of HCC tissue-specific autophagy-based target therapy system (AFP-Cre/LoxP-shRNA). (A) The AFP-Cre/LoxP-shRNA system consisted of AFP-Cre vector and LoxP-shRNA vector. A CMV-eGFP reporter in the system could indicate whether the system worked. (B) In vitro analysis showed that the system worked efficiently in HCC cells (HCCLM3/HepG2) but did not work in non-HCC cells (L-02/Hela). The disappearance of GFP fluorescence indicated that the Cre recombinase driven by the AFP promoter had cut the LoxP-CMV-eGFP-LoxP in U6 promoter and activated expression of shRNAs targeting BECN1 and ATG5. Western blot analysis showed that the system efficiently silenced target genes BECN1 and ATG5. Cre+LoxP or Cre+LoxP NC: cotransfection of AFP-Cre and LoxP-shRNA (AFP-Cre/LoxP-shRNA system); NC, negative control; AFP-Cre or LoxP-shRNA: transfection of AFP-Cre or LoxP-shRNA alone (control). (C) In vivo analysis showed that intratumoral injection of AFP-Cre/LoxP-shRNA system in HCCLM3 subcutaneous tumors worked efficiently in vivo.
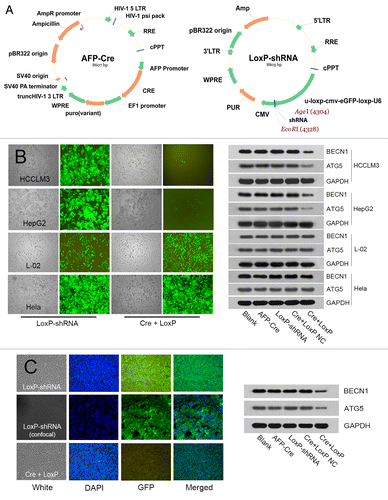
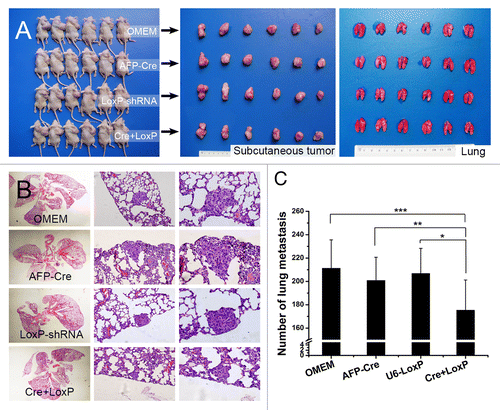
Figure 8. In vivo evaluation of HCC-tissue-specific autophagy-based target therapy for HCC metastasis. (A) A nude mouse model of pulmonary metastasis via subcutaneous injection was established. The target therapy system was intratumorally administrated. Cre+LoxP: AFP-Cre/LoxP-shRNA system; OMEM: Opti-MEM (control); AFP-Cre: AFP-Cre alone (control), LoxP-shRNA: LoxP-shRNA alone (control). From left to right: mice bearing subcutaneous HCCLM3 tumors, subcutaneous tumors of the mice, lungs of the mice; (B and C) The number of lung metastases in the Cre+LoxP group was significantly less than those in the other 3 groups (*P = 0.015, **P = 0.022, ***P = 0.007). (From left to right) representative panoramic image of lung section, lung metastasis (100×), and lung metastasis (200×).