Figures & data
Figure 1. PROM1/CD133+ cells of colorectal cancer exhibit the characteristics of CSCs and resistance to PDT. (A) The PROM1/CD133+ cells were isolated from PCC or HT29 by FACS sorting. The percentage of PROM1/CD133+ cells was determined by FACS assay. (B) The morphology of PROM1/CD133+ cells was observed 10 d after FACS sorting. The PROM1/CD133+ cells formed floating tumorspheres under stem-cell conditions in the presence of EGF and FGF2. Scale bar: 100 μm. (C) Total RNA was isolated from PROM1/CD133− and PROM1/CD133+ cells from PCC and HT29 cells and then analyzed by qPCR for SOX2, OCT4, NANOG, and CTNNB1. (D) Following PpIX-mediated PDT at various light doses, PROM1/CD133+ and PROM1/CD133− cells viability was analyzed by ANXA5-FITC-PtdIns staining at 24 h after PDT. These results are expressed as the mean ± SE of 3 different experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
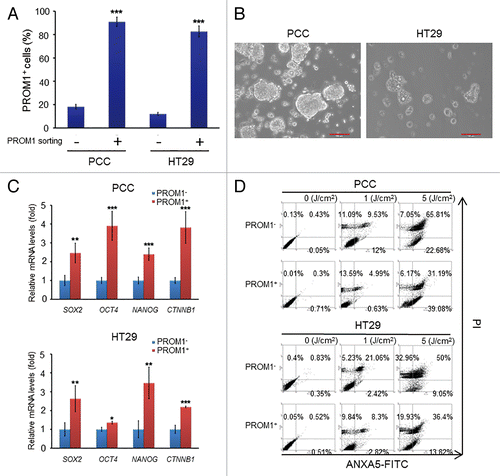
Figure 2. PpIX-mediated PDT leads to an increased level of autophagy in PROM1/CD133+ cells. (A) PROM1/CD133− and PROM1/CD133+ cells were treated with PDT (1.3 J/cm2). After 24 h, total cell lysates were analyzed by western blot. The band intensities on films were analyzed by ImageJ software. The relative amounts of LC3-II were quantified as ratios to ACTB, indicated underneath each gel. The relative ratio of LC3-II in PROM1/CD133− cells without PDT treatment is arbitrarily presented as 1. (B) Cells were labeled with anti-LC3 primary antibody and DyLight 488 conjugated secondary antibody at 24 h after PDT (1.3 J/cm2). LC3 puncta were observed by immunofluorescence using confocal microscopy. Nuclei were counterstained with DAPI. Scale bar: 10 μm. The number of LC3 puncta was counted using 20 cells for each condition. (C) Flow cytometric analysis of AO staining for cells at 24 h post PDT treatment (1.3 J/cm2). These results are expressed as the mean ± SE of 3 different experiments. *P < 0.05.
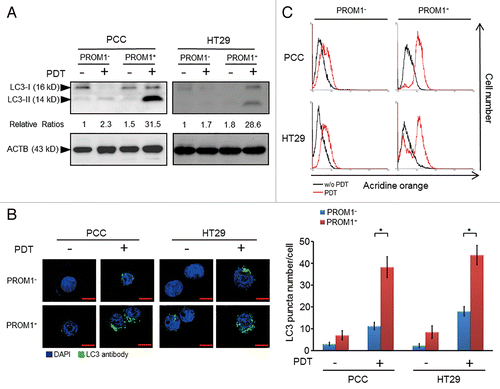
Figure 3. PpIX-mediated PDT combined with autophagy inhibitors enhanced the cytotoxic effect and blocked colonosphere formation in CSCs. (A) The PROM1/CD133+ and PROM1/CD133− cells form PCCs and HT29 cells were treated with chloroquine (10 μM) or 3-MA (5 mM) for 24 h followed by PDT at various light doses. Cytotoxicity was measured using the WST-1 assay. (B) and (C) The disaggregated PROM1/CD133+ cells were plated at 100 cells/well in an ultra-low attachment plate and treated with PDT (1.3 J/cm2) in the presence of CQ or 3-MA. Colonospheres that contained at least 20 cells were counted in 8 different wells. Representative images show the sizes of the newly formed colonospheres after 7 d. Scale bar: 50 μm. The results are expressed as the mean ± SE of 3 different experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
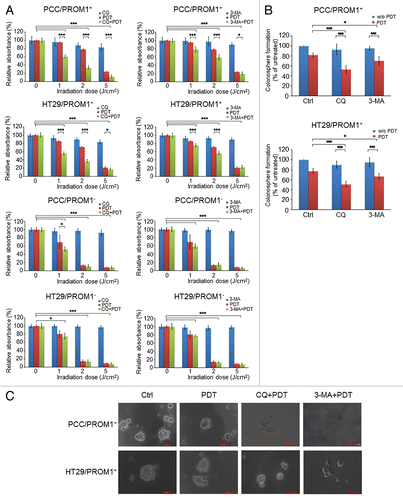
Figure 4. Genetic silencing of autophagy enhances the cytotoxic effect of PpIX-mediated PDT in the CSCs. (A) The total extracts of PROM1/CD133− and PROM1/CD133+ cells were harvested at 24 h post-PDT (1.3 J/cm2). The levels of ACTB and autophagy-related proteins, including ATG3, ATG5, ATG7, ATG12, and BECN1, were assessed by western blot analysis. The band intensities on films were analyzed by ImageJ software. The relative amounts of each protein were quantified as ratios to ACTB, indicated underneath each gel. The relative ratio of each protein in PROM1/CD133− cells is arbitrarily presented as 1. (B) The PROM1/CD133+ PCCs were infected with pGIPZ lentivirus (scrambled), ATG3 or ATG5 shRNA lentivirus. The protein expression of ATG3 and ATG5 was examined by western blot analysis. (C) The PROM1/CD133+ PCCs and PROM1/CD133+ HT29 cells that expressed specific shRNA were treated with PDT at various light doses. The cytotoxicity was then measured using the WST-1 assay. (D and E) The disaggregated PROM1/CD133+ PCCs that expressed specific shRNA were plated at 100 cells/well in an ultra-low attachment plate and treated with PDT (1.3 J/cm2). The number and size of the colonospheres were assessed as described for . Scale bar: 50 μm. The results are expressed as the mean ± SE of 3 different experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
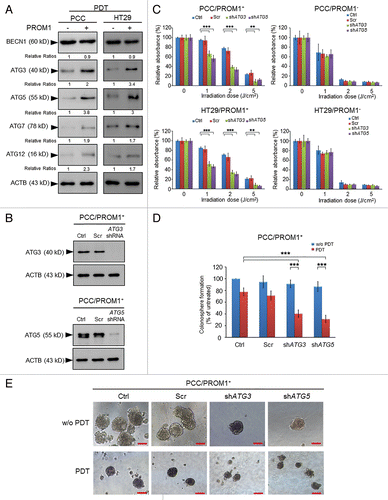
Figure 5. Inhibition of autophagy by chloroquine or ATG5 shRNA enhanced the apoptotic effect of PDT in CSCs. (A) Apoptotic cells of PROM1/CD133+ PCCs were visualized by TUNEL assay at 24 h post-PDT (1.3 J/cm2). Nuclei were specifically labeled using DAPI staining. Scale bar: 50 μm. The percentage of TUNEL-positive cells was scored per 100 cells. (B) Whole cell lysates of PROM1/CD133+ PCCs were collected at 24 h post-PDT (1.3 J/cm2) and measured by CaspACE™ Assay System for CASP3 activities. (C) The percentage of apoptotic cells of PROM1/CD133+ PCCs was monitored by ANXA5-FITC-PtdIns staining and flow cytometry at 24 h post-PDT (1.3 J/cm2), and apoptotic ratio was calculated by plotting ANXA5-FITC-positive cell number against total cell number. These results are expressed as the mean ± SE of 3 different experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
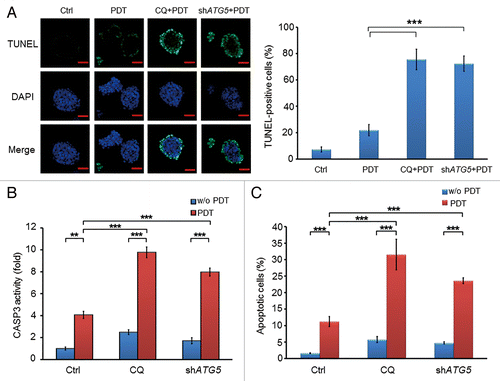
Table 1. Tumor-initiating potential of PROM1/CD133+ PCCs in each treatment group
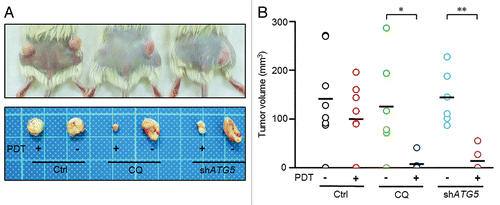
Figure 6. Inhibition of autophagy enhanced the antitumorigenicity of PDT in a xenograft model. (A) A total of 1 × 103 viable PROM1/CD133+ PCCs, pretreated with CQ (10 μM), ATG5 shRNA lentivirus, or PDT (1.3 J/cm2), either alone or in the indicated combinations, were implanted subcutaneously into both flanks of NOD/SCID mice. Representative pictures of each treatment group. Mice were sacrificed 12 wk after implantation. (B) Tumor sizes were measured at the end of the experiment. Data are presented as dot plots, and the short black lines indicate mean tumor size. Tumor volume was calculated as (L * W2)/2, where L is the length and W is the width of the tumor. The tumors in CQ-treated and ATG5-silenced groups were significantly distributed in lower tumor volume ranges with PDT compared with without PDT. *P < 0.05; **P < 0.01.