Figures & data
Figure 1. C16-ceramide treatment induces EMD phosphorylation. (A) HCT116 cells were transfected with GFP siRNA or EMD siRNA and then stimulated with C16-ceramide (12 µM) for the indicated times. EMD was revealed by western blotting using an anti-EMD antibody. An anti-TUBA1A antibody was used as an internal control. (B) EMD mRNA levels, extracted from HCT116 cells treated with and without C16-ceramide, were assessed by quantitative real-time PCR analysis.
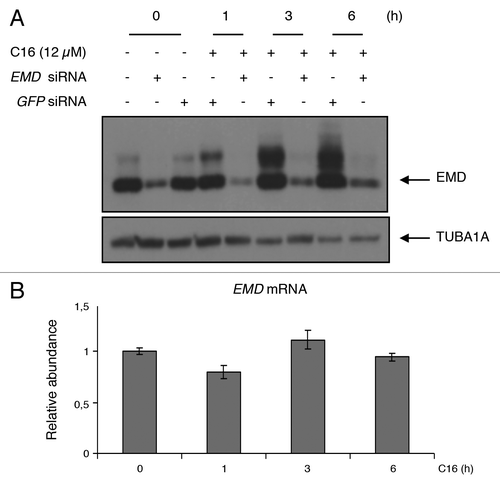
Figure 2. Phosphatase (λPPase), general kinase inhibitor (staurosporine), and PRKACA inhibitor (H89) reverse EMD modification. (A) Colon cancer cells were treated with or without C16-ceramide for 1, 3, and 6 h. Protein extracts were incubated in the absence or presence of alkaline phosphatase (λPPase) at 30 °C for 30 min. Protein extracts were separated on SDS polyacrylamide gel and immunoblotting was revealed with anti-EMD and anti-TUBA1A antibodies. (B) HCT116 cells were pretreated with staurosporine (100 nM) for 30 min and left untreated or stimulated with C16-ceramide (12 µM) for 1, 3, and 6 h. Western blotting analysis was performed using an anti-EMD and an anti-TUBA1A antibody. (C) HCT116 were pretreated with the PRKACA inhibitor, H89 (5 µM) for 1 h and treated with or without C16-ceramide for the indicated periods of time. Cell extracts were subjected to anti-EMD and anti-TUBA1A antibodies for western blot analysis.
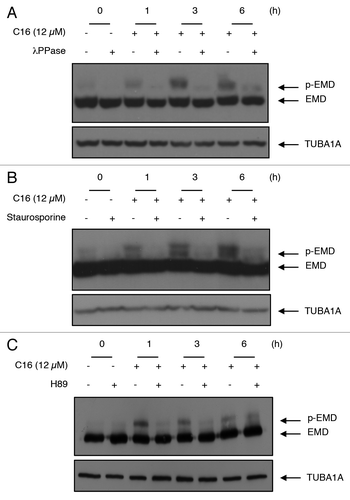
Figure 3. C16-ceramide induces EMD phosphorylation on its LEM domain. (A) Schematic representation of full-length EMD and mutants (LEMΔ, N74Δ, N105Δ). (B) HCT116 cells were transfected with EMD-FLAG, LEMΔ-FLAG, N74Δ-FLAG, N105Δ-FLAG, or empty vector and subjected to C16-ceramide for 3 h. Cell extracts were analyzed with an anti-FLAG western blot.
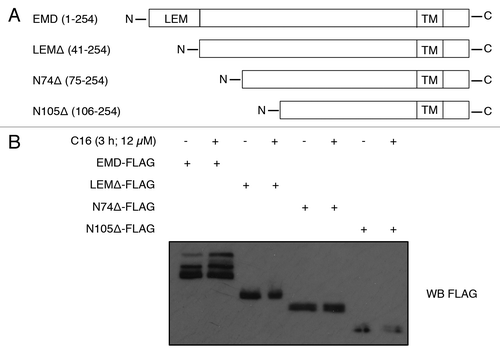
Figure 4. C16-ceramide treatment induces lethal autophagy in HCT116 cells. (A) HCT116 were treated with C16-ceramide for the indicated periods of time. Cell lysates were analyzed by immunoblotting with anti-MAP1LC3B and -TUBA1A antibodies. (B) HCT116 were pretreated with bafilomycin A1 (100 nM) for 3 h and treated with or without C16-ceramide. Cell lysates were analyzed by immunoblotting with anti-MAP1LC3B and -TUBA1A antibodies. (C) HCT116 cells were transfected with GFP siRNA or ATG7 siRNA. ATG7 and MAP1LC3B were revealed by western blotting using anti-ATG7 and anti-MAP1LC3B antibodies. The anti-TUBA1A antibody was used to detect TUBA1A as an internal control (top). Viability test. HCT116 cells were transfected with GFP siRNA or ATG7 siRNA and then treated with and without C16-ceramide for 3 h. Cell viability was measured using the WST1 assay (bottom). (D) Cells were treated with or without C16-ceramide and cell lysates were analyzed with anti-AKT1, -pAKT1, -MTOR, -pMTOR, -RPS6KB1, -pRPS6KB1, and -TUBA1A antibodies.
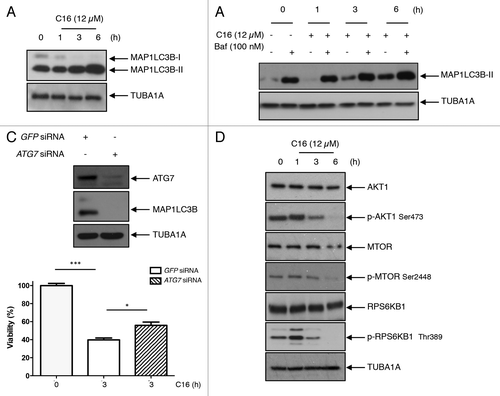
Figure 5. Implication of EMD in the C16-ceramide autophagic pathway (A) HCT116 cells were transfected with EMD-FLAG or empty vector and then left untreated or stimulated with C16-ceramide for the indicated times. Cell extracts were assayed using anti-MAP1LC3B, -EMD and -TUBA1A antibodies for western blotting analysis (left). The intensity of each band corresponding to MAP1LC3B-II was measured with the Quantity One software (Bio-Rad). Data are representative of 3 independent experiments and P values were calculated using t tests (*P < 0.05; **P < 0.001; ***P < 0.0001) (right). (B) HCT116 cells were transfected with GFP siRNA or EMD siRNA and then stimulated with C16-ceramide (12 μM) for the indicated times. MAP1LC3B was revealed by western blotting using an anti-MAP1LC3B antibody. Anti-EMD and anti-TUBA1A antibodies were also used. (C) Cells of each condition were stained with anti-MAP1LC3B antibodies and analyzed with confocal microscopy. Representative fluorescent images (left). Statistic results of the percentage of cells presenting a punctate distribution of MAP1LC3B (right).
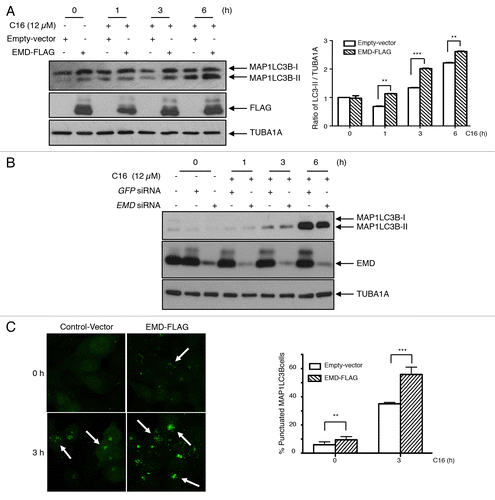
Figure 6. EMD binds MAP1LC3B upon ceramide treatment. (A) Cells were transfected with EMD-FLAG or with empty vector and then treated with or without C16-ceramide for 3 h. After immunoprecipitation of FLAG, MAP1LC3B was revealed by western blotting using an anti-MAP1LC3B antibody. (B) Representative fluorescent images of HCT116 cells untreated or treated with C16-ceramide for 3 h and stained with both anti-EMD and -MAP1LC3B antibodies. Cells were analyzed with confocal microscopy.
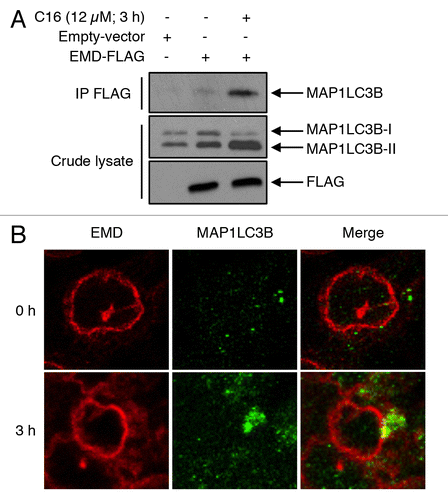
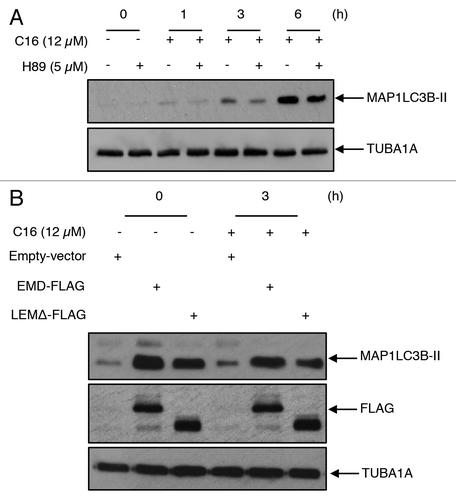
Figure 7. EMD induces autophagosome formation through its phosphorylation triggered by C16-ceramide (A) HCT116 were transfected with EMD-FLAG vector or with empty vector. Cells were then pretreated with the PRKACA inhibitor, H89 (5 µM) for 1 h and treated with or without C16-ceramide for 1, 3, or 6 h. Cell lysates were analyzed by western blotting using an anti-MAP1LC3B antibody. (B) Cells were transfected with EMD-FLAG, LEMΔ-FLAG, or empty-vector and then treated with ceramide for 3 h. MAP1LC3B, FLAG, and TUBA1A were revealed by western blotting using anti-MAP1LC3B, anti-FLAG and anti-TUBA1A antibodies.