Figures & data
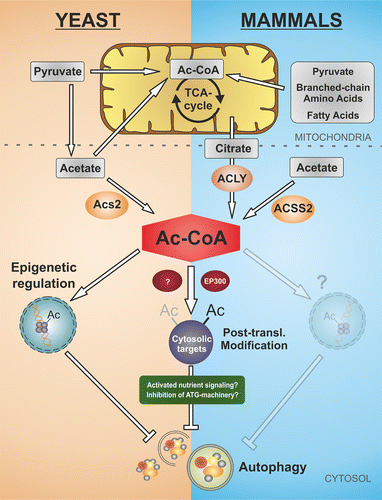
Figure 1. Nucleo-cytosolic AcCoA determines the autophagic response in yeast and mammals. In Saccharomyces cerevisiae grown on glucose, the major source for nucleo-cytosolic AcCoA is the AcCoA synthetase 2 (Acs2), which uses acetate as a substrate. Mitochondria solely influence cytosolic AcCoA levels by removing acetate and its precursor pyruvate, fueling the tricarboxylic acid (TCA) cycle. In contrast, cytosolic AcCoA production in mammals requires a detour via mitochondria, since it depends on citrate derived from the TCA cycle, which is fed by pyruvate as well as by fatty acids and branched-chain amino acids. Citrate is exported from mitochondria and converted to AcCoA by the cytosolic ATP citrate lyase (ACLY). In both models, high concentrations of nucleo-cytosolic AcCoA favor protein acetylation by acetyltransferases. Protein hyperacetylation subsequently inhibits autophagy by epigenetic regulation of autophagy-related genes, by direct posttranslational inactivation of proteins engaged in the autophagic machinery, as well as by (direct or indirect?) modulation of nutrient-sensing kinase pathways.