Figures & data
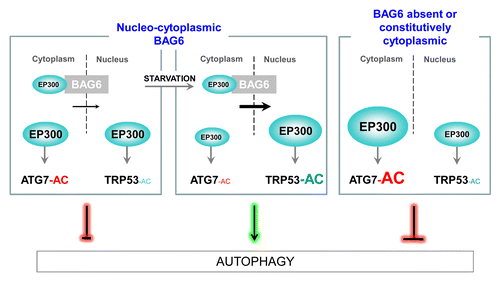
Figure 1. BAG6 inhibits EP300-dependent acetylation (AC) of ATG7 but stimulates the nuclear acetylation of TRP53 during starvation. In the cytosol, BAG6 inhibits ATG7 acetylation, an inhibitory event in autophagy, by limiting the quantity of the acetyltransferase EP300 available in the cytosol. On the contrary, by favoring the nuclear transport of EP300 under starvation conditions, BAG6 i) stimulates the nuclear TRP53 acetylation and ii) maintains a low level of ATG7 acetylation, both leading to the stimulation of autophagy. When BAG6 is absent or constitutively located into the cytoplasm, autophagy is inhibited. Concomitantly, a strong accumulation of cytoplasmic EP300 occurs, leading to the hyperacetylation of ATG7 and the inhibition of TRP53 acetylation upon starvation.