Figures & data
Figure 1. GTP-GDP exchange cycle of RAB proteins. RABs cycle between GTP-bound active and GDP-bound inactive forms. In their active, membrane-attached state they recruit various effectors. GTPase-activating proteins (GAPs) increase the GTP hydrolysis rate, thereby inactivating RABs. Inactive RABs are sequestered in the cytosol by GDP dissociation inhibitors (GDIs). Upon RAB activation, GDI displacement factors (GDFs) can displace GDIs. Afterwards, guanine nucleotide exchange factors (GEFs) activate RAB proteins by changing GDP to GTP.
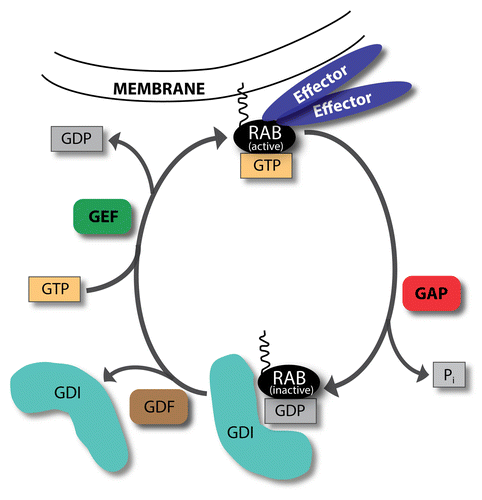
Table 1. RAB proteins implicated in autophagy
Figure 2. Various roles of RAB7 in autophagy. Through its interaction partners, RAB7 is implicated in the bidirectional transport of autophagosomes. RILP participates in the recruitment of dynein-dynactin complex, whereas FYCO1 interacts with kinesins. Other RAB7 effectors, UVRAG, KIAA0226/Rubicon, and RNF115 have a role in autophagosome maturation. While the HOPS complex associated SNAREs, VTI1B, and VAMP8, are involved in autophagosome-lysosome fusion, the HOPS itself, although a well-known RAB7 effector, is not implicated in this process. Furthermore, RAB7 is implicated in the regulation of TORC1 activity, and, together with its effector PLEKHF1/Phafin1, is also described in autophagosome (AP) formation.
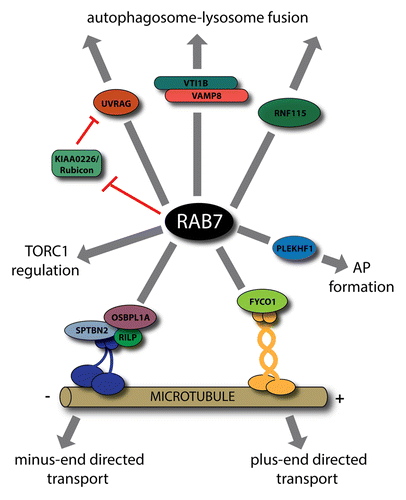
Table 2. Rab GEFs and GAPs and their role in autophagy
Figure 3. RAB1/Ypt1 has a key role in autophagosome formation. Through its interactors, the conserved oligomeric Golgi (COG) complex and Atg11, it facilitates ATG9/Atg9 cycling. Moreover, activated by the RAB1 GEF, TRAPPIII, RAB1 participates in the recruitment of ULK1/Atg1 to the PAS.
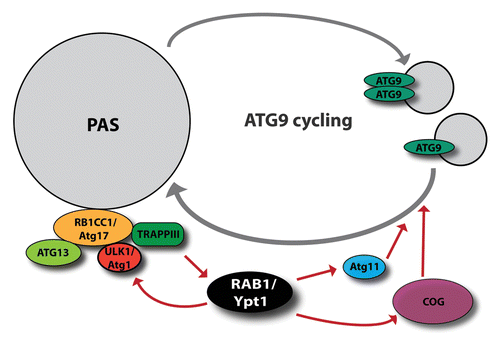
Figure 4. Roles of RAB11 in the regulation of autophagy. RAB11 seems to have different roles in distinct steps of autophagy. Together with TBC1D14 it plays a role in membrane trafficking from the recycling endosome (RE) to the PAS. SNX18 is also implicated in this process. Furthermore, upon autophagy induction by starvation, RAB11 removes Hook—a negative regulator of endosome maturation—from LEs allowing subsequent fusion of a LE with an autophagosome (AP).
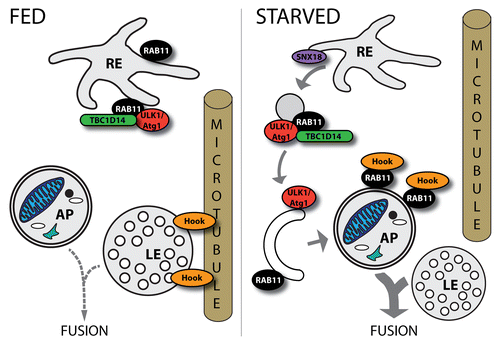
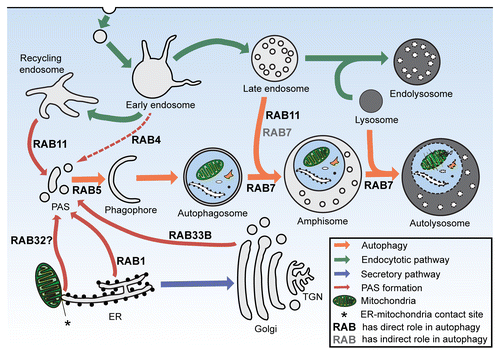
Figure 5. The functions of RAB proteins in autophagy. The schematic picture shows the interactions of autophagy with the endocytic and secretory pathways. A set of RAB small GTPases play a role in the earliest steps of autophagosome formation by providing various membrane sources for the PAS. RAB5 also participates in this autophagic stage, through its interactions with the PIK3C3-BECN1 complex. RAB7 has both direct roles—in the transport of autophagosomes and amphisomes—and indirect roles in the fusion process of autophagosomes with late endosomes. Besides its roles in PAS formation, RAB11 regulates amphisome formation and coordinates the autophagic and endosomal pathways. ER, endoplasmic reticulum; TGN, trans-Golgi network; PAS, phagophore assembly site.