Figures & data
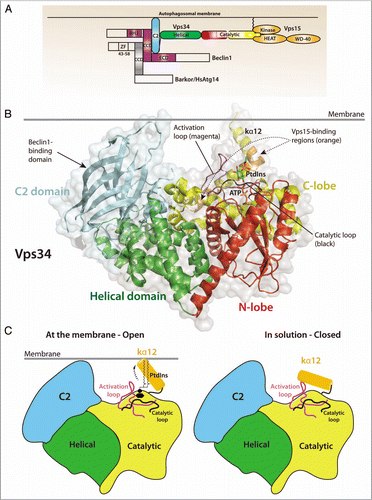
Figure 1 (A) Domain organization of Vps34, its regulatory subunit Vps15 and the adaptor proteins required for autophagy induction in mammalian cells, Beclin 1 and Atg14L/Barkor (Beclin1-associated autophagy-related key regulator). (B) Structure of Drosophila Vps34 helical (green) and catalytic (red/yellow) domains. A PtdIns substrate molecule has been modeled between the activation loop (magenta) and the catalytic loop (black) and ATP was modeled based on the p110γ/ATP structure (PDB ID 1E8X). The C2 domain (cyan) was also modeled from the p110γ/ATP structure. The enzyme is oriented so that the C2 domain and C-terminal helix interact with the membrane. Two regulatory proteins bind directly to Vps34: Vps15 binds to helices kα11 and kα12 (orange), and Beclin 1 binds to the C2 domain. Both Vps15 and Beclin 1 stimulate Vps34 activity. (C) A schematic representation of the Vps34 domains and the putative change in conformation of the kα12 helix. In solution (right), the helix is closed and interacts with residues in the substrate-binding and catalytic loops to exclude water. At the membrane (left), the kα12 helix undergoes a conformational change and interacts with the membrane, enabling productive substrate binding and catalysis.