Figures & data
Figure 1 Analysis of lipopolysaccharide structure in E. coli Nissle 1917 derived strains. The LPS of wild-type E. coli Nissle 1917 (EcN), msbB-mutant E. coli Nissle 1917 (ΔmsbB), or each strain containing the O-antigen encoding pBWB536 plasmid, was isolated and separated by gel electrophoresis. Subsequent silver gel staining revealed the presence of the core region and in case of pBWB536 containing bacteria, the O-antigen. The strains lacking the msbB gene had a reduced core size indicated by arrows.
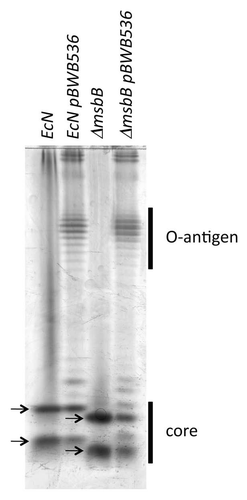
Figure 2 Growth of wild-type and msbB-mutant E. coli Nissle 1917. (A) E. coli Nissle 1917 wild-type (EcN) and msbB-mutant (αmsbB) were grown at 37°C in LB broth and no differences between the two strains were observed. (B) In contrast, growth of the wild-type bacteria on MSB-agar was inhibited by deoxycholate and only 0.5% of the plated cfu could be recovered while about 80% of the msbB-mutant bacteria formed colonies.
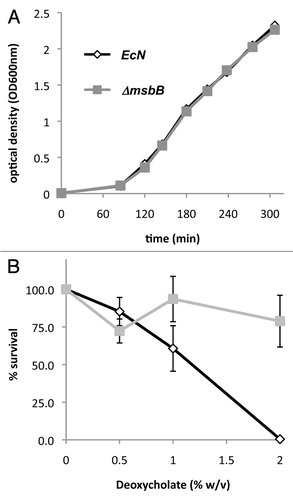
Figure 3 Induction of the proinflammatory cytokines TNFα and IL-6 in murine macrophages. Cells of the murine macrophage cell line J774 were co-incubated for 4 h with 2 × 104 or 2 × 106 cfu of wild-type (EcN, dark grey) and msbB-mutant (ΔmsbB, light grey) E. coli Nissle 1917, respectively. The formation and secretion of TNFα (A) and IL-6 (B) was then determined in the supernatant. Supernatants of cells treated with medium alone served as control and background levels (118 pg/ml for TNFα and 0 pg/ml for IL-6) were substracted from the obtained results. The data show significantly lower amounts of both proinflammatory cytokines when treated with the msbB-mutant strain.
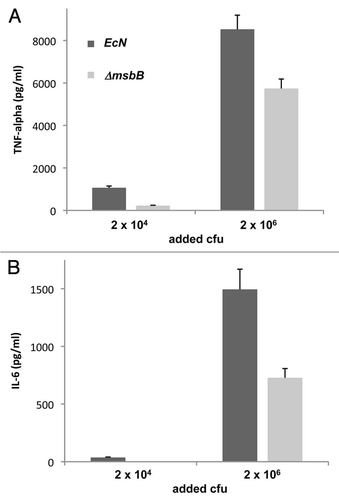
Figure 4 LD50 of E. coli Nissle 1917 strains in BALB/c mice. Different amounts of wild-type (EcN) and msbB-mutant (ΔmsbB) E. coli Nissle 1917 were i.p. injected into BALB/c mice. Latter bacterial strain resulted in reduced toxicity and therefore higher survival rates (about 10-fold higher LD50) when compared to the wild-type strain.
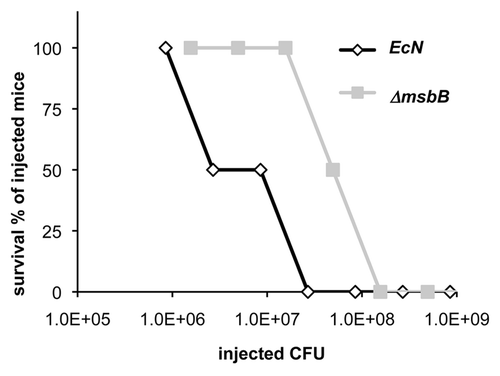
Figure 5 Tumor colonization of E. coli Nissle 1917 strains. BALB/c mice bearing 4T1 tumors were intravenously injected with 1 × 106 cfu of wild-type and msbB-mutant E. coli Nissle 1917, respectively. In addition, the bacteria of the same strains were injected which harbored the plasmid pBWB536. The plasmid leads to the synthesis of O-antigen conferring serum resistance. Two days post injection, tumor, liver and spleen of each mouse were isolated and analyzed for the presence of bacteria. Each strain was able to selectively colonize the tumor tissue with almost no background levels in spleen and liver.
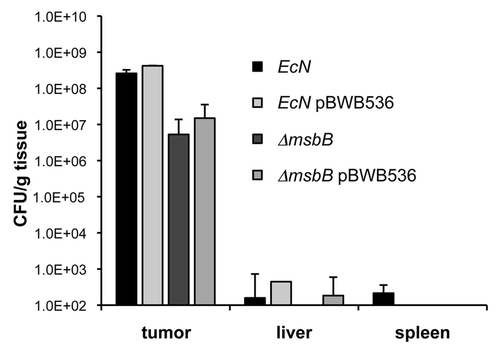
Table 1 Primers used in this study